Resources
AVAC’s Resource Database contains educational and advocacy materials covering a wide range of issues on biomedical prevention of HIV, STIs, COVID-19 and emerging health threats—from research to rollout.
To search for clinical trials and detailed information on products in development, visit our Prevention Research & Development Database

Results
showing 1-10 of 314
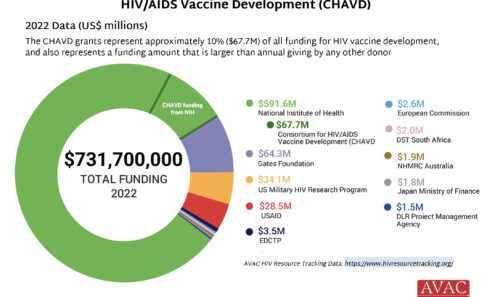
HIV Prevention Product Overview
The graphic provides an overview of PrEP products currently available and in late-stage clinical trials.
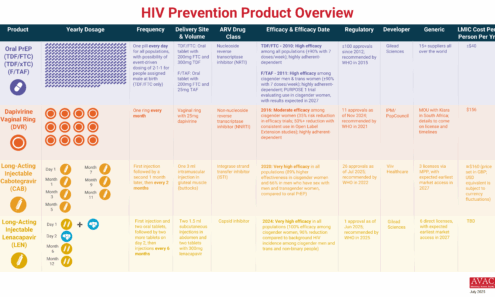
Years Ahead in HIV Prevention Research: Time to Market
This timeline shows the potential time points when the next-generation of HIV prevention options might find their way into new programs.
Prevention Option:
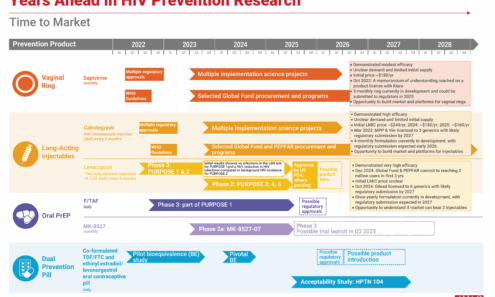
Potential Demand for LEN for PrEP
The top 16 PrEP markets, based on 2024 oral PrEP initiations and the possible 2026 injectable market, assuming 60% of new PrEP users choose an injectable, as seen in implementation studies.
Prevention Option:
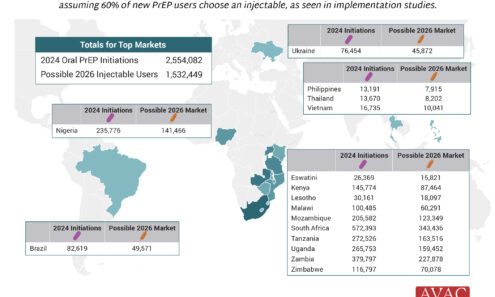
Impact of Cuts to the Consortia for HIV/AIDS Vaccine Development (CHAVD)
In May 2025, NIH’s National Institute of Allergy and Infectious Diseases (NIAID) announced that funding for the Consortia for HIV/AIDS Vaccine Development (CHAVD) would end after the current grant cycle in June 2026. With only one more year of funding before the grants end, current plans for research, clinical trials and progress toward a vaccine are all at risk.
Prevention Option:
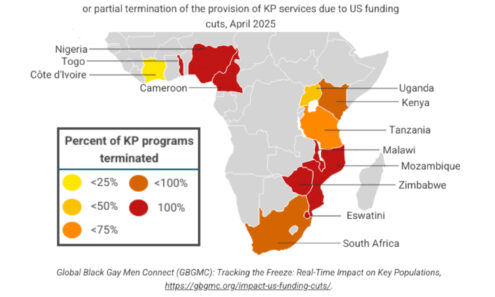
Impact of US Funding Cuts on Services for Key Populations
Percentage of key population-serving implementing partners that have reported full or partial termination of the provision of KP services due to US funding cuts (as of April 2025).
Prevention Option:
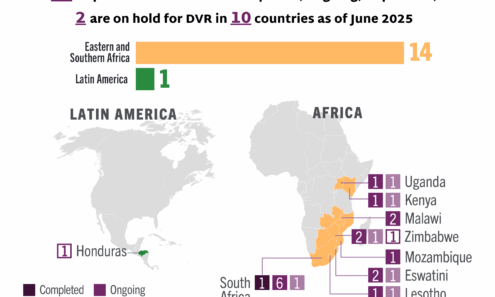
Dapivirine Vaginal Ring Implementation
Ongoing and planned implementation studies for the dapivirine vaginal ring as of June 2025. For product approvals, volumes, implementation, and price comparisons of long-acting PrEP, visit our dashboard on PrEPWatch.org.
Prevention Option:
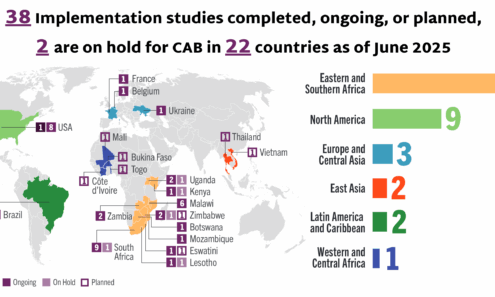
Cabotegravir Implementation
Implementation studies completed, ongoing, or planned for cabotegravir as of June 2025. For product approvals, volumes, implementation, and price comparisons of long-acting PrEP, visit our dashboard on PrEPWatch.org.
Prevention Option:
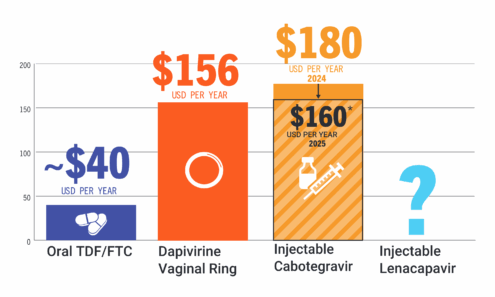
PrEP Price Comparison
Comparing the annual price of oral TDF/FTC vs. the dapivirine vaginal ring and injectable cabotegravir. For product approvals, volumes, implementation, and price comparisons of long-acting PrEP, visit our dashboard on PrEPWatch.org.
Prevention Option:
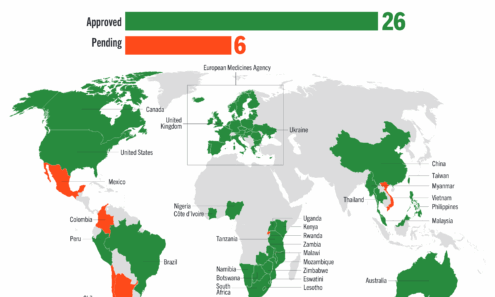
Dapivirine Vaginal Ring Regulatory Approval
Regulatory approvals, pending decisions, and appeals as of June 2025. For product approvals, volumes, implementation, and price comparisons of long-acting PrEP, visit our dashboard on PrEPWatch.org.
Prevention Option:
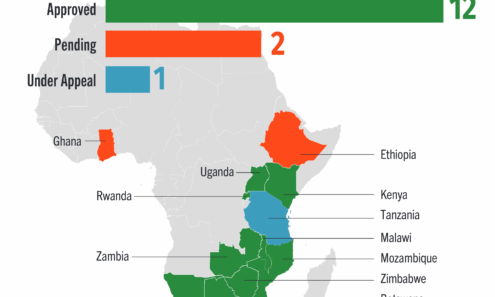
Cabotegravir Regulatory Approval
Regulatory approvals and those pending for cabotegravir as of June 2025. For product approvals, volumes, implementation, and price comparisons of long-acting PrEP, visit our dashboard on PrEPWatch.org.
Prevention Option:
showing 1-10 of 314