Resources
AVAC’s Resource Database contains educational and advocacy materials covering a wide range of issues on biomedical prevention of HIV, STIs, COVID-19 and emerging health threats—from research to rollout.
To search for clinical trials and detailed information on products in development, visit our Prevention Research & Development Database

Search or Filter
Results
showing 1-10 of 931
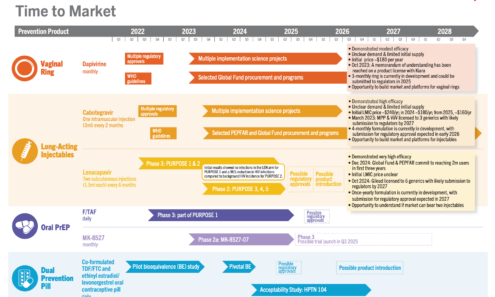
Years Ahead in HIV Prevention Research: Time to Market
This timeline shows the potential time points when the next-generation of HIV prevention options might find their way into new programs.
Prevention Option:
Lawsuit Wins and What’s at Stake
On February 10, AVAC led other organizations to sue the US government including the President, the US State Department and USAID, seeking emergency relief from an Executive Order that inhumanely froze all funding for foreign assistance. This case may well help to determine the future of foreign assistance, executive overreach, and the role of evidence, facts, and values in US policy.
AVAC’s Executive Director, Mitchell Warren and Public Citizen Litigator, Lauren Bateman explain these lawsuits and why they matter.
Better Engagement, Better Evidence
Writing in The Lancet Global Health, AVAC staffers Stacey Hannah, Jessica Salzwedel, and several co-authors, write about the importance of community stakeholder engagement clearly seen after World Health Organization adoption of new rules requiring clinical trials to improve this kind of coordination.
Topic:

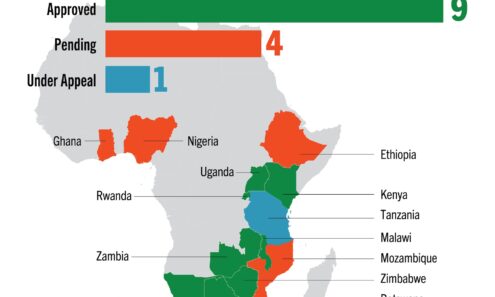
Dapivirine Vaginal Ring Regulatory Approval
Regulatory approvals, pending decisions, and appeals as of March 2025. For product approvals, volumes, implementation, and price comparisons of long-acting PrEP, visit our dashboard on PrEPWatch.org.
Prevention Option:
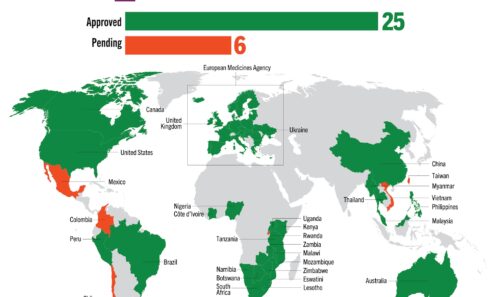
Cabotegravir Regulatory Approval
Regulatory approvals and those pending for cabotegravir as of March 2025. For product approvals, volumes, implementation, and price comparisons of long-acting PrEP, visit our dashboard on PrEPWatch.org.
Prevention Option:
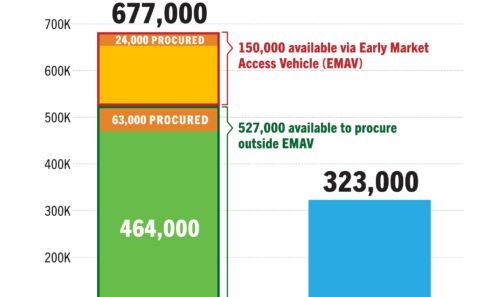
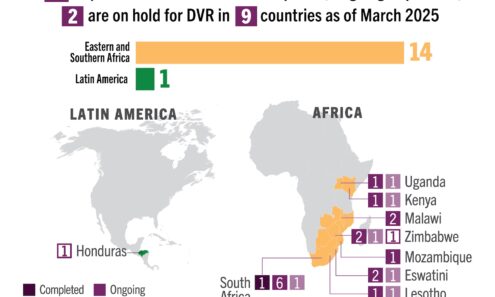
Dapivirine Vaginal Ring Volume
DVR supply available to low- and middle-income countries as of March 2025. For product approvals, volumes, implementation, and price comparisons of long-acting PrEP, visit our dashboard on PrEPWatch.org.
Prevention Option:
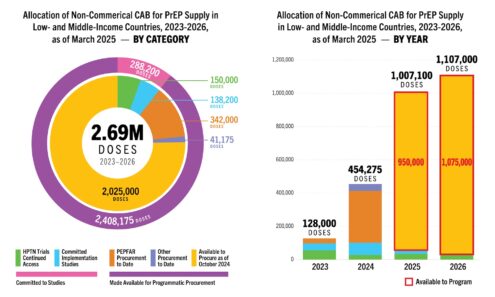
Cabotegravir Volume
Allocation of non-commercial cabotegravir for PrEP supply in low- and middle-income countries, 2023-2026, as of March 2025. For product approvals, volumes, implementation, and price comparisons of long-acting PrEP, visit our dashboard on...
Prevention Option:
Dapivirine Vaginal Ring Implementation
Ongoing and planned implementation studies for the dapivirine vaginal ring as of March 2025. For product approvals, volumes, implementation, and price comparisons of long-acting PrEP, visit our dashboard on PrEPWatch.org.
Prevention Option:
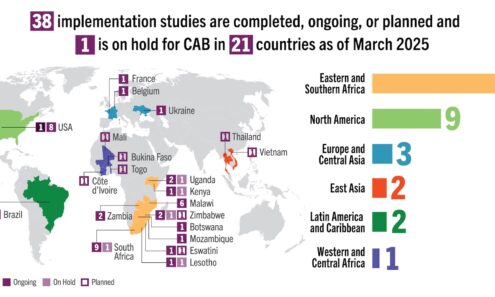
Cabotegravir Implementation
Implementation studies completed, ongoing, or planned for cabotegravir as of March 2025. For product approvals, volumes, implementation, and price comparisons of long-acting PrEP, visit our dashboard on PrEPWatch.org.
Prevention Option:
Self-Care Advocacy for HIV and STI Prevention
Self-care is especially critical now, as the new US administration’s sweeping funding cuts and policy shifts threaten to erode support for traditional healthcare services, including HIV and STI programs. By putting testing, prevention, and treatment directly into people’s hands, self-care can help communities maintain vital health services despite reduced funding, limited access to healthcare, and diminished government support.
Prevention Option: