COVID-19 may have forced some limiting factors onto the 11th IAS Conference on HIV Science—the conference was primarily online; the attendance was lower; and we couldn’t interact with our colleagues face-to-face—but moving in and out of virtual sessions, something transformative was in the air. The intersection of the responses to COVID-19 and HIV and the glaring disparities in health made ever more undeniable by COVID-19, are giving new meaning and momentum to changes advocates have been pressing for years, even decades. Where to begin…
- DSD: The time is now
- The latest on the prevention pipeline
- And vaccines?
- COVID-19 and HIV
- PrEP and resistance
- Calls for people-centered solutions
- New targets, old problems
DSD: The time is now
Differentiated service delivery (DSD) accelerated by orders of magnitude—telephone counseling, dispensing multi-month supplies of drugs, home delivery, community-based refills, and other innovation staved off hundreds of thousands of predicted HIV deaths, which were expected if people couldn’t get to the pharmacy or the clinic for treatment during COVID-19 lockdowns.
Multiple conference sessions referred to numerous initiatives where DSD scaled up rapidly, and with success. A few examples: An initiative from the CQUIN network coordinated among 21 different countries to share resources and lessons on DSD. PEPFAR’s Catherine Godfrey reported from a review of 52 countries that found 30 of them had scaled up and expanded eligibility for multi-month dispensing for treatment, with a 79 percent increase in the total number of people receiving a six-month supply of drugs. A different review from seven PEPFAR-supported countries, showed overall treatment interruption did not increase. And a UNAIDS analysis showed global trends in ART initiation continued despite the lockdown. Crucial to these successes, said Godfrey, were community health workers and community-based drug distribution.
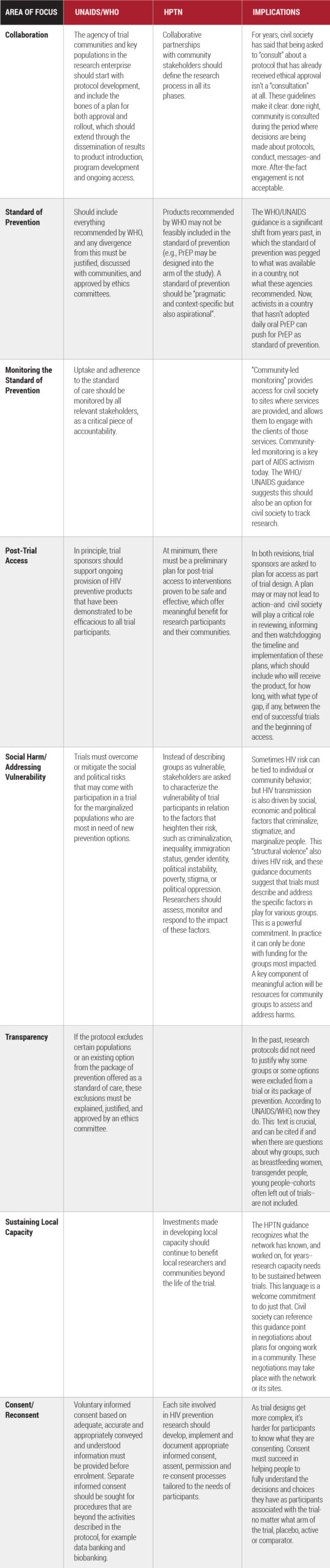
These lessons from antiretroviral treatment have been applied in prevention as well. The session, Paving the road for new PrEP products: The promise of differentiated, simplified, and decentralized delivery to maximize the potential of new products, expanded the discussion on the need for innovations in PrEP delivery. Panelists at the satellite session, convened by AVAC, IAS and PATH, noted delivery must evolve as new products move through the pipeline, or years will be wasted with low uptake. Simplifying and Improving PrEP Delivery is one of four issue briefs just published by the Prevention Market Manager project and launched at the satellite, along with Reframing Risk in the Context of PrEP, Generating and Sustaining Demand for PrEP, and Next Generation M&E for Next-Generation PrEP. All echo core principles embedded in DSD: center the development of programs, policies and products in the leadership and knowledge of those who need prevention most.
This movement to DSD holds tremendous potential for prevention just as the field is poised to add powerful new prevention options to the tool kit. Data on next-generation options were also presented at IAS.
The latest on the prevention pipeline
Days before IAS began, The HealthTimes reported Zimbabwe’s approval of the monthly Dapivirine Vaginal Ring (DVR), which would be the first country to do so. Data presented at IAS on ring use from the REACH study underscore what can be achieved by tailoring the design of products and programs. REACH is a study looking at safety and adherence of oral PrEP and the ring in young women ages 16-21. Data from the study showed a marked increase in adherence to both the ring and oral PrEP among adolescent girls compared to previous studies. The key factor in the uptick? Intensive, individualized support that allowed participants to select from a menu that included text messages, weekly check-ins by phone, “peer-buddy” and adherence support groups. These findings build on evidence from other studies, including the POWER study, that adolescents greatly benefit from intensive adherence support. REACH data on preferences between the ring and oral PrEP are forthcoming.
A ring-focused session—Intravaginal options: Today and in the future—covered a lot of ground, from early-stage research on multipurpose rings for prevention, to work the World Health Organization (WHO) is doing to help facilitate the introduction of the Dapivirine Vaginal Ring. The WHO already recommends the ring and its Coordinator of Treatment and Care in the Department of HIV/AIDS, Meg Doherty, said the WHO plans to develop tools and guidance to assist countries to fit this new tool into their context, work on the supply chain, generate demand and more. These tools and guidance are expected by early 2022.
On the research front, Andrea Thurman (CONRAD) presented data from Phase I studies of a ring loaded with levonorgestrel (LNG) and tenofovir (TFV) and showed it is safe, acceptable and met benchmarks for drug levels in the body. Further research is warranted. Another presentation in that session came from Sharon Hillier (UPMC Magee-Womens Hospital), who presented highlights from the pipeline of intravaginal ring research including a three-month Dapivirine Ring and a novel MPT ring that’s both non-hormonal and includes a non-ARV-based antiviral.
A satellite session, Bringing the Dual Prevention Pill to Market: Opportunities for HIV and Pregnancy Prevention and Implications for Future Multipurpose Prevention Technologies (MPTs), reviewed the status of the Dual Prevention Pill, which could be available as early as 2024. If approved, it would be the first MPT to go to market since male and female condoms. This novel strategy provides unprecedented opportunities to integrate sexual and reproductive health (SRH) with HIV services, but major challenges must be met. The field of women’s health and family planning, policy makers and all stakeholders will need to understand why and how HIV prevention is their priority too, and how the DPP fits in the context of community health. Early, frequent and comprehensive consultations across both fields can and must serve as a model for breaking down these silos.
In a late-breaker session, Sharon Hiller presented data from the Phase 2a study of monthly oral islatravir, building on data presented earlier this year at HIVR4P. Hillier’s presentation at IAS 2021 showed this strategy met—and exceeded—key benchmarks for drug levels in the body that researchers think will be needed for it to be effective. The week-24 data also showed that it’s safe and well-tolerated. Efficacy data on this new prevention option will come from two large-scale efficacy trials (IMPOWER-22 and IMPOWER-24), results of which are expected in 2024. Islatravir is also being studied for long-term use via an implant.
And vaccines?
With a robust non-vaccine prevention pipeline, some of the vaccine session titles seemed to hint at a field wondering about its place: Will there be a next generation HIV vaccine if current ones fail?; An HIV vaccine: who needs it?; and HIV vaccines and immunotherapy: Quo vadis? (Latin for “Where are you going?”) The answer to that question is that HIV vaccine research is moving forward. Discussions at these sessions pointed to intensifying global interest in vaccine research and implementation, with COVID on everyone’s mind—and much of the success in COVID vaccines owed to the legacy of research in HIV. HIV vaccine research is moving forward with diverse approaches, and efficacy data from the two ongoing large-scale trials of Janssen’s Adeno 26-based vaccine are expected within the next two years. The AMP trials, and their implications for vaccine and antibody research, were also part of the discussion and are discussed in more detail in our recent document, Understanding Results of the AMP Trials.
But this momentum can’t be taken for granted. As Lynn Morris, from the University of the Witwatersrand and a leading antibody researcher, said in her plenary, it’s important now to galvanize political will, support increased investment and industry involvement in HIV vaccine development, run more parallel trials and take more risk.
And no matter the challenges of vaccine science, once available people will need to take it. AVAC’s Daisy Ouya reminded attendees at a UNAIDS/HIV Vaccine Enterprise satellite session that early and consistent community engagement is essential. If the field is going to reach coverage targets, community mistrust can’t be a barrier.
COVID-19 and HIV
A WHO report found HIV is a risk factor for severe or critical COVID-19. The WHO Global Clinical Platform for COVID-19 drew data from 24 countries and more than 15,000 people. This report offers another picture on HIV and COVID from the largest pool of data to date compared to other smaller studies that did not find a link, including one from the US, also presented at IAS 2021. The results have reinforced the need to ensure people living with HIV are listed as priority populations to receive COVID vaccines across the globe. This must happen in the context of massive scale-up to produce and distribute vaccines and close the gap in vaccination between the global north and south.
PrEP and resistance
The GEMS project monitored for the development of drug resistance among PrEP-takers. The project analyzed samples from 104,000 people from PrEP programs in Eswatini, Kenya, South Africa and Zimbabwe. There were 229 documented seroconversions overall and 118 were sequenced (some participants didn’t provide samples and some samples had low viral load and couldn’t be sequenced). Of those 118, 23 percent had HIV with a PrEP-associated mutation for drug resistance. Urvi Parikh, Senior Project Advisor of GEMS, presented the data and put the findings in context: the number of reported infections while on PrEP was very small, and an even smaller number had resistant mutations.
Based on the GEMS data, the most common mutation, M184V/I, is not expected to undermine the efficacy of one of the most important treatments across Africa, dolutegravir-based regimens. It will be important to monitor where these mutations will affect future treatment. It will also be vital to carefully screen for HIV among people who want PrEP in order to catch early infections that could lead to resistance. For additional background, our colleague Gus Cairns did a terrific summary of these findings as part of the NAM/AIDSMAP reports throughout the conference.
Calls for people-centered solutions
IAS 2021 heard an emphasis on the importance of community-led, client-controlled empowerment and leadership, which starts at the beginning of the research process, framing the questions that must be answered and how to answer them.
No Data No More: Manifesto to Align HIV Prevention Research with Trans and Gender Diverse Realities launched during IAS 2021. Developed by trans and gender-diverse (TGD) advocates from South Africa, Europe and the United States, with support and solidarity from AVAC, the manifesto demands an HIV prevention research agenda based on the priorities of TGD communities, with those communities driving decisions throughout the process.
Among the demands: provide gender-affirming hormone treatment (GAHT) across the continuum of HIV research, prevention and care; address the barriers that limit access to research and prevention for TGD people; recruit TGD researchers and other experts to design and implement solutions at all phases of the response.
At a session, What is missing in the HIV response?: Strengthening HIV programmes for trans populations in the Global South, presenters decried the absence of data on trans health outcomes even as TGD people face high risk of HIV. See US CDC statistics that show 1 in 7 (14 percent) transgender women in the United States have HIV and an estimated 3 percent of transgender men have HIV, with even less data available for non-binary individuals.
Liberty Matthyse of Gender Dynamix in South Africa said “trans and gender-diverse healthcare is greatly understudied,” fundamentally undermining the effort to end the epidemic. “We need to prioritize community-led participatory research, plug into existing community-led movements, co-create context-specific methodology to reach missing and undocumented cases. And we need to decentralize access to hormonal care and make it part of a package of HIV services. Every person should have the right to gender self-determination.”
Rena Janamnuaysook’s presentation at this session provided a model. At Thailand’s Tangerine Clinic, members of the trans community identified priority health needs, co-designed a program of services, and received training to deliver qualified care. About 4,000 community members rely on the clinic, which offers hormone services and other gender-affirming care as an anchor to generate demand for other health services including HIV testing, prevention and treatment. Nearly all (94 percent) of the 10 percent who have tested positive have initiated ART, nearly all of them (97 percent) are virally suppressed, and 18 percent of those who have tested negative initiated PrEP. “Key populations-led services increased access to testing, simplified services, and promoted a sustainable HIV response,” said Rena.
New targets old problems
These stories from IAS 2021 put the past year in perspective. The science that was needed to respond to both SARS-CoV2 and HIV has seen significant advances and must continue. The innovation to deliver treatment and prevention has also seen unprecedented effort, and must accelerate and expand. But policy and political will has failed to close gaping inequities that mean, among other things, the 3.3 billion doses of COVID vaccine delivered to date are concentrated in a few wealthy countries, that lockdown drove up reports of rape in Uganda by 24 percent while the use of PEP dropped by 17 percent, that 150 million people will fall into extreme poverty this year, that COVID remains a threat to global health everywhere.
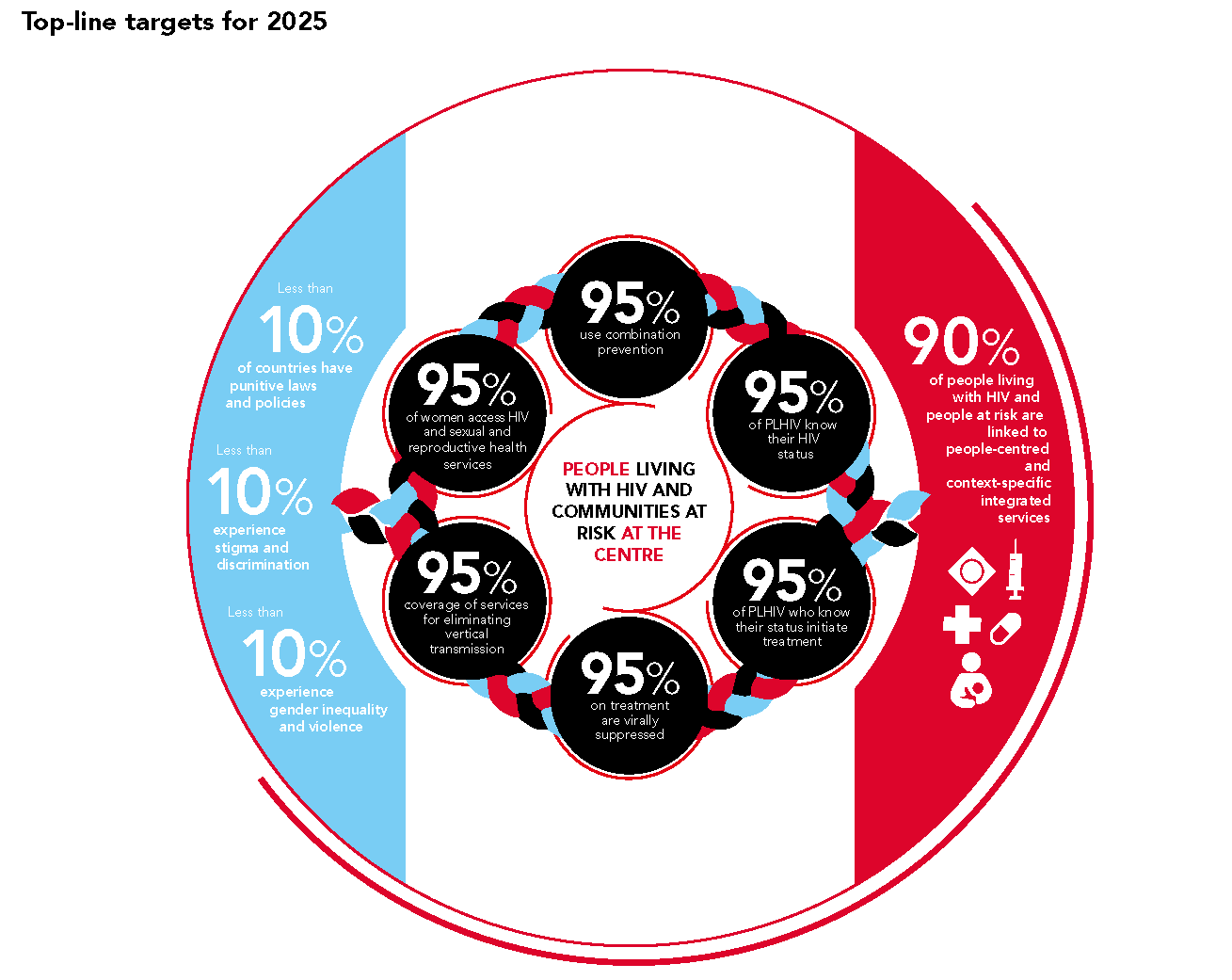
The new UNAIDS targets, the so called “10’s” aimed at social factors that drive epidemics (punitive laws and policies, stigma, discrimination and gender-based violence) should galvanize action from every quarter. Approved by the UN’s High Level Meeting on HIV in June, these new targets must be used to build on the sweeping changes underway in 2020, to dismantle structural barriers and to embrace new evidence-based models that will make prevention a reality everywhere it’s needed.