October 4, 2024
We learned of progress this week in building a sustainable market for choice in prevention with the news of generic licensing agreements for lenacapavir as injectable PrEP. We are moving faster than the first decade of oral PrEP and the beginning rollout of injectable cabotegravir, which is an example of advocacy at its best, but making lenacapavir and new prevention options available to all who need it, requires even greater speed, scale and equity. Coordination and strategic actions among numerous and varied stakeholders are urgently needed, as outlined in this article from the New York Times.
See AVAC’s updated Lens on LEN, our new Plan for Accelerating Access to Injectable Lenacapavir for PrEP, along with additional resources on LEN!
From Clinical Trial Efficacy to Public Health Impact: A Plan for Accelerating Access to Injectable Lenacapavir for PrEP
This plan provides a comprehensive view of all the moving parts and identifies priority actions and actors responsible for ensuring time is not wasted and opportunity not squandered.
The Lens on LEN: The basics on injectable lenacapavir as PrEP
This updated advocates’ primer with the PURPOSE 2 data provides background on lenacapavir and its trials, raises key questions, and explores next steps.
Lenacapavir: The case for investing in delivering HIV prevention

This episode of PxPulse goes deep on LEN for PrEP. Recorded just days before Gilead’s announcement that PURPOSE 2 also found very high efficacy, Dr. Flavia Kiweewa, a principal investigator of PURPOSE 1, the first trial to announce efficacy, lays out the research findings and what they mean. And Chilufya Kasanda Hampongo of Zambia’s Treatment Advocacy and Literacy Campaign and Mitchell Warren of AVAC talk about how to change a long history of squandered opportunities to get rollout right.
Breaking Ground: Expanding Access to Lenacapavir

This webinar hosted by UNITAID brought together global health experts, community advocates, and civil society organizations to discuss the challenges and opportunities in ensuring equitable access to Lenacapavir.
An Overview of Lenacapavir PrEP Trials
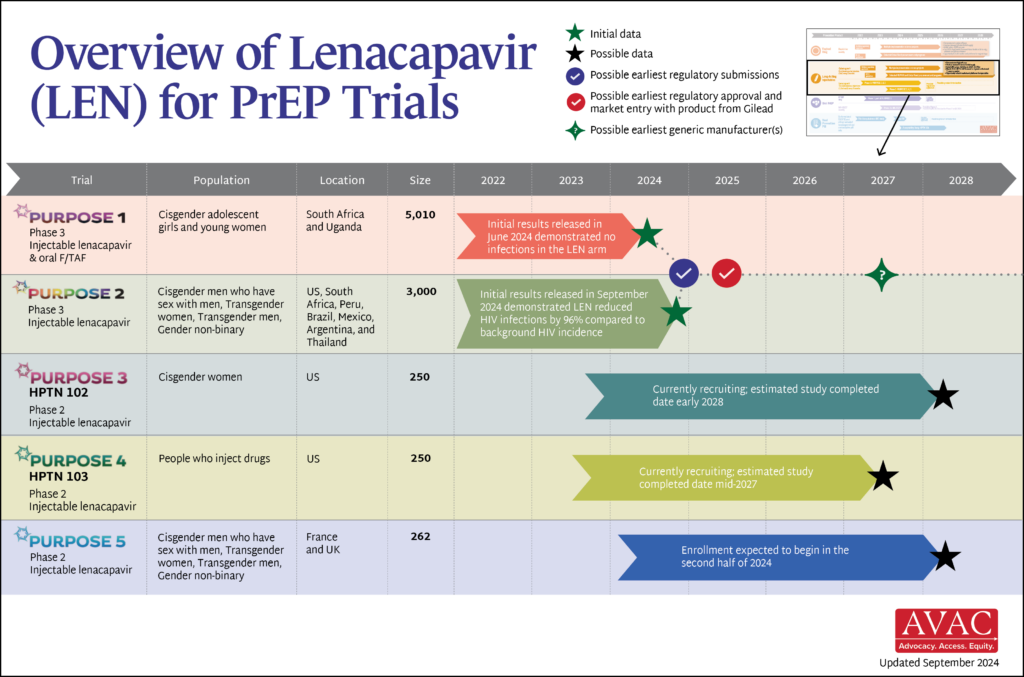
The PURPOSE trials evaluate the safety and efficacy of injectable lenacapavir (LEN), an investigational antiretroviral (ARV) drug being studied as a potential PrEP product. This graphic shows the latest status of all five trials including the groundbreaking results of PURPOSE 1 and PURPOSE 2.
Gilead Agrees to Allow Generic Version of Groundbreaking HIV Shot in Poor Countries
This news New York Times article raises key questions on access and pricing of lenacapavir. “Mr. Warren said he hoped the major global health agencies would work together to place orders large enough to have at least a million people on lenacapavir by the end of 2026, which would help send a clear signal to generics makers about the potential market for the product.”