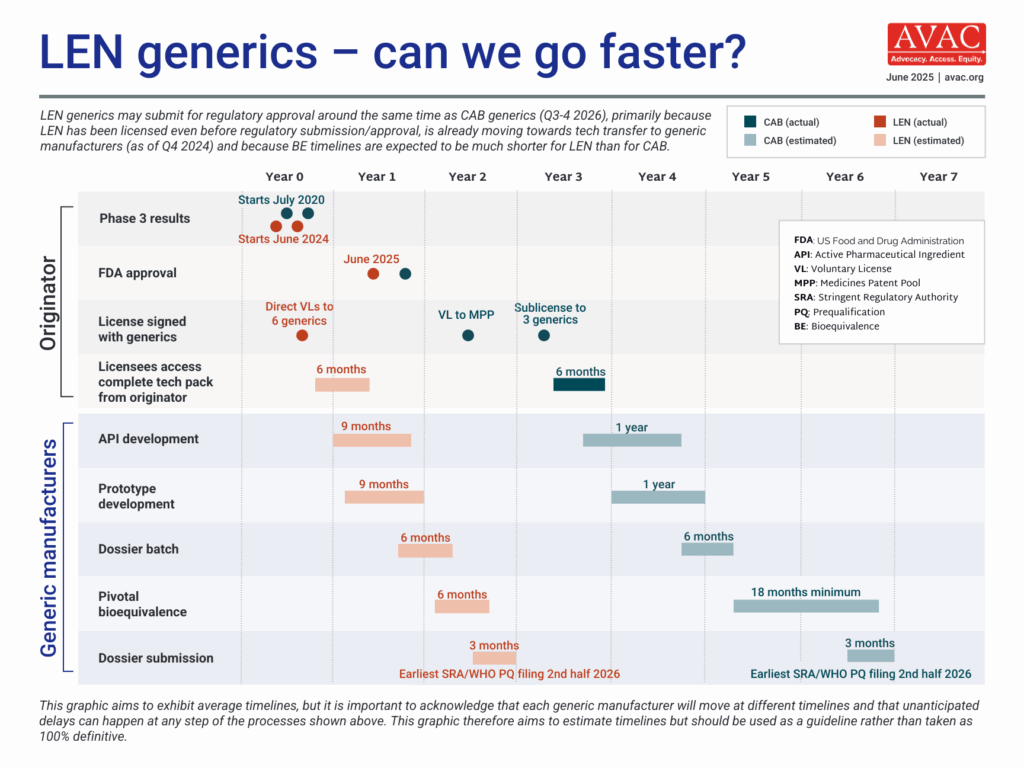
The timeline for generic LEN for PrEP to come to market is expected to be significantly shorter than for CAB for PrEP. Bioequivalence (BE) testing for LEN, which demonstrates a generic product works in the body in the same way as the originator product, is likely to be six months, vs. the 18 months for CAB for PrEP, because of differences in the drug formulation. The rapid granting of voluntary licensing by Gilead also contributes to this shorter timeline. For the latest on LEN, visit here.
- Topics:
- Advocating for Health Equity
Was this content helpful?
Tell us how we can improve the content.
Was this content helpful?
Thank you for your feedback!