December 2, 2024
This World AIDS Day, the HIV/AIDS response stands at a crossroads, with injectable lenacapavir set to transform HIV prevention. But as the new UNAIDS report highlights, it also comes at the same time as restrictive policies, economic instability, and geopolitical challenges threaten to frustrate access and rollback so much of the progress that has been achieved over the past two decades.
AVAC’s 2025 advocacy agenda prioritizes collaboration and strategies for equitable and accelerated product introduction that maximize the public health potential of new prevention options and simultaneously sustains investment in critical research and development. AVAC’s newest publication, The Gears of Lenacapavir for PrEP Rollout, provides a clear pathway for the speed, scale and equity needed to translate exciting science into public health impact, while our recent The People’s Research Agenda (PRA) meets this high-stakes moment for HIV prevention with a clear, concise and collaboratively developed set of priorities for how prevention research should be conducted and what products should be developed in the future.
Despite the challenges, 2025 holds immense potential for ensuring the equitable rollout of new options and the accelerated development of a pipeline of additional options, the combination of which can help move the field closer to ending HIV/AIDS.
For the latest information on injectable lenacapavir for PrEP, be sure to join us for our webinar Tuesday, December 3. And read more below about both the Gears of LEN for PrEP Rollout and The People’s Research Agenda.
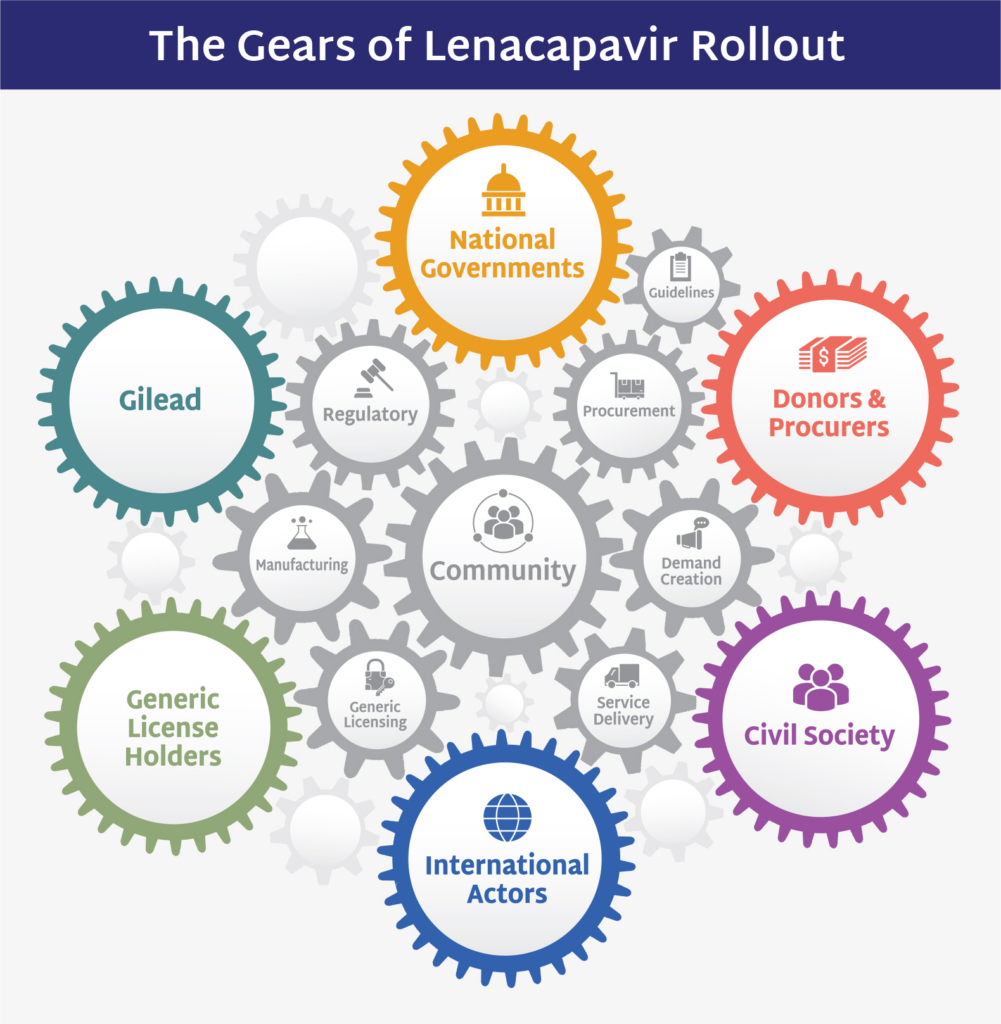
The Gears of Lenacapavir for PrEP Rollout: Driving Speed, Scale, and Equity
Lenacapavir’s rollout is not just about making a new drug available as quickly as possible; it is about ensuring that it reaches the people who need it most, as swiftly and equitably as possible. Gilead has announced its readiness to manufacture up to 10 million doses for 2026, but this potential hinges on coordinated action by governments, donors, and civil society. The roadmap outlines the essential gears driving this effort, from robust demand generation and procurement strategies to equitable distribution and community-driven implementation. Crucially, the roadmap emphasizes lessons learned from previous PrEP interventions: that availability alone is not enough. With global HIV targets still unmet and disparities persisting, this effort demands decisive action and long-term planning.
The People’s Research Agenda: A Community-Driven Vision
The People’s Research Agenda (PRA) brings the voices of affected communities to the forefront of HIV prevention research and product development. With limited resources, the stakes for decisions about which products to develop and eventually deliver become even higher for funders, communities, policy makers and governments. The PRA offers a bold vision for aligning scientific innovation with community needs. By amplifying the perspectives of those most affected by the epidemic, the PRA is a tool for driving accountability among funders, developers, and policymakers. As a living, adaptable framework, it ensures that evolving needs and challenges in the prevention landscape remain at the center of decision-making.

A Call to Action
The rollout of lenacapavir and the implementation of the PRA come at a critical moment in the global HIV/AIDS response. Achieving their full potential will require sustained collaboration, strategic investments, and unwavering commitment to equity. Together, we can transform this pivotal moment into lasting progress.