March 13, 2025
The science offered at the Conference on Retroviruses and Opportunistic Infections (CROI) 2025 is a showcase of the great promise and importance of research. With broadside attacks and a sweeping funding withdrawal by the US government affecting science and global health, these advances in the field make clear how much is at stake. Innovation in long-acting PrEP could transform global health, new evidence to support on-demand PrEP among women, insights coming from cure research, and expanding efforts to confront an epidemic in sexually transmitted infections (STI), if the field can unite behind the evidence and refuse to be defeated.
New data presented Monday underscores these dire realities: nearly one in five children under one with HIV who experienced a treatment interruption in 2024 died, based on a review of over half a million children in US-funded PEPFAR programs. These findings emphasize the critical need to maintain uninterrupted care for young children with HIV, who are especially vulnerable to rapid disease progression, and starkly highlight the catastrophic risks associated with halting funding for treatment programs.
Read on for more important scientific highlights.
The Power of LEN for PrEP
A once-yearly injection of lenacapavir (LEN) for PrEP took a step forward. Renu Singh of Gilead Sciences presented data from an ongoing, open-label study of the pharmacokinetics (how a drug is absorbed, distributed and eliminated in the body at a given dose), safety and tolerability of two LEN injections testing an intramuscular formulation. Singh reported that drug concentrations were as good as or better than the 6-monthly subcutaneous dose that showed 100% efficacy in 2024.
“It gives me a great pleasure to see a small molecule last this long. It’s never been done before. Once yearly injectable LEN for PrEP has the potential to offer high efficacy.”
The doses were safe and well tolerated. A Phase 3 study is expected to launch later this year, with possible regulatory submissions in 2027. Gilead’s application for twice-yearly LEN for PrEP is currently under FDA priority review with a decision expected by June 19. (ViiV Healthcare is similarly advancing their four-month injectable cabotegravir (CAB) towards possible regulatory submission, while the two-month formulation is rolling out.)
More data from the PURPOSE Trials were presented showing that a cohort of 16-17 year-old females were just as well protected by 6-month injections of LEN for PrEP as adults in the larger study. Katharine Gill of the Desmond Tutu HIV Foundation, South Africa credited the PURPOSE trials and Good Participatory Practice for the landmark study, the first large-scale trial to include adolescents in the initial study design. Despite having high HIV incidence, adolescents have historically been excluded from Phase III HIV trials, resulting in prolonged delays in access to PrEP.
“PURPOSE 1 shows us that working with community and including adolescent stakeholders, we can design ethical studies that are effective and inclusive, especially for young women who need prevention the most,” said Gill.

On Demand PrEP for Cis-gender Women: 3 days or 4?
Findings from a modeling study explored how to optimize an on-demand protocol for HIV protection in the female genital tract. On-demand PrEP, sometimes called event-driven PrEP or 2-1-1, is a CDC approved protocol for men who have sex men who opt to take PrEP over a three-day period, starting 2-24 hours before the time of a specific sexual exposure, then 1 pill every 24 hours for the following two days. Mackenzie Cottrell of the University of North Carolina reported findings that adding a fourth day of dosing heightened protection from vaginal exposure to HIV.
Dosing of 2-1-1-1 showed 84% protection, 2-2-2-2 showed 95% protection. Cottrell said, “While limited data suggest 2-2-2-2 dosing is safe for short-term use, the 2-1-1-1 regimen may better balance safety, efficacy, and tolerability while maintaining effectiveness” Cottrell recommended the 2-2-2, 2-1-1-1, and 2-2-1-1 regimens be considered in future clinical studies of on-demand PrEP in cisgender women.
This data builds on the analysis of a body of evidence, led by Jeanne Marrazzo, now director of NIAID, showing that oral PrEP can reliably prevent HIV infection in cisgender women even with non-perfect adherence.
MK-8527: A Monthly PrEP Pill
Merck presented an analysis of animal and Phase I data that informed the MK-8527 doses chosen to be tested in its ongoing Phase II PrEP trial. Looking at protection provided in monkeys, as well as pharmacokinetic data in humans, Merck determined that a monthly dose of at least 6 mg would provide adequate protection in more than 90% of the population intended for MK-8527 use. The Phase II trial is testing 3mg, 6mg, and 12mg doses in participants with low risk of HIV exposure. Watch this space for further information about how the Phase II trial will inform the upcoming efficacy program of MK-8527 expected to start later this year.
F/TAF Works in Women With Med-High Adherence
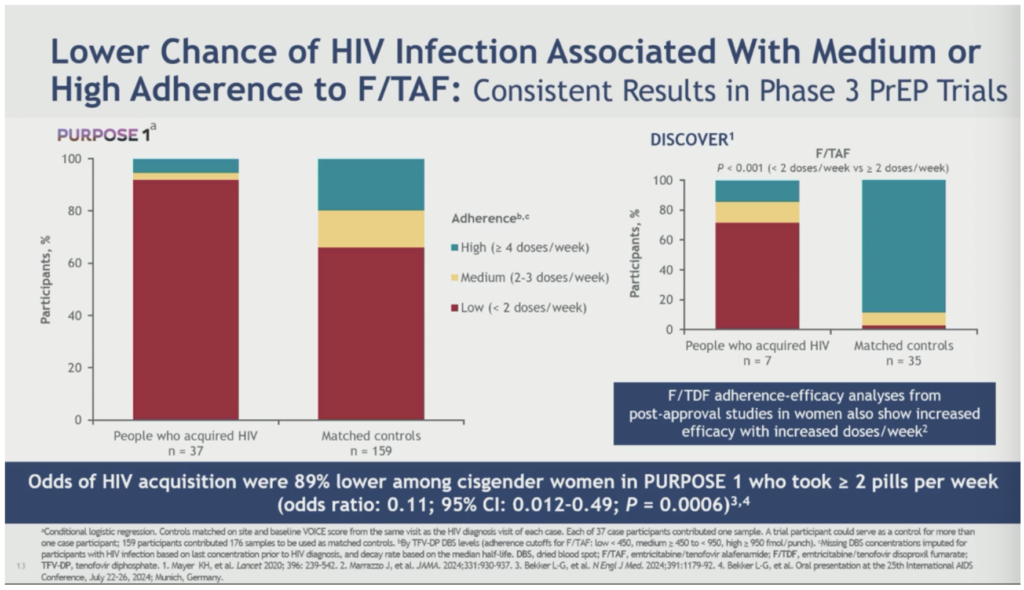
Flavia Kiweewa of Makerere University-Johns Hopkins University Research Centre in Uganda presented important new prevention evidence from the PURPOSE 1 trial that tested both injectable LEN and F/TAF among cisgender women for PrEP. This new analysis found the chance of acquiring HIV was 89% lower when adherence to F/TAF reached two pills per week or more.
Kiweewa reported that “nearly all incident HIV cases in participants receiving F/TAF in PURPOSE 1 were attributable to low oral PrEP adherence… taken together, these results suggest that HIV infections in PURPOSE 1 occurred almost always in the context of nonadherence to F/TAF, with rare emergence of HIV resistance and low risk of HIV diagnosis delay.” Kiweewa concluded that F/TAF is another prevention tool that should be considered for women who prefer a daily oral HIV prevention option.
Advances in Cure Research
A key focus for cure research at this year’s CROI was data from the FRESH Cohort in South Africa looking at delivery of the CAP 256 broadly neutralizing antibody (bNAb) in combination with TLR9, an immune activator developed by Gilead Sciences, to women identified and treated extremely early in infection. The trial involved an analytical treatment interruption (ATI) and allowed for eight weeks of consecutive viral loads above 1,000 copies before treatment was restarted. This allowed the researchers to see interesting viral dynamics, including about a third of the women experiencing multiple periods of viremia off therapy, some even getting close to 100,000 copies/mL in blood. The viral loads quickly returned to undetectable (after re-starting therapy?). Four women in the trial continue to remain off therapy.
Investigators from another key cure study, the RIO study, shared results that bNAbs at the time of treatment initiation were able to achieve durable control. Twenty-one individuals had delayed rebound with seven experiencing rebound over one year. The numbers are small, but combined with other basic and clinical trial data, bNAbs are an intervention to watch—for possible prevention, treatment and cure!
The Latest on DoxyPEP
While doxyPEP is already in use as an STI prevention intervention in several countries, ongoing research continues to address key questions regarding antimicrobial resistance, adherence, and identifying the populations that would benefit most. The PRIDOX study looked at the impact of doxyPEP in “high-risk MSM” who were also using PrEP in a real-world setting. Christina Gómez-Ayerbe presented data showing that the 197 study participants who started doxyPEP saw lower incidence of syphilis, gonorrhea and chlamydia at the end of the study than the incidence rates at the beginning of the study before starting doxyPEP. And similar to most doxyPEP studies, there were higher rates of gonorrhea than the other bacterial STIs studied. Even among participants who contracted gonorrhea while on doxyPEP, no cases of drug-resistant strains or other forms of microbial resistance were observed—a notable finding given that some other studies have reported modest increases in AMR among doxyPEP users.
Another study from Milan, Italy, looked at doxyPEP use among MSM using the U.S. CDC’s doxyPEP guidelines (which suggest prescribing doxyPEP for MSM and transgender women who have had one STI in the past 12 months). It found that out of 251 participants, 85 infections could have been prevented with doxyPEP, yet 164 individuals would have been unnecessarily prescribed it since they had no further STIs. Dr. Roberto Rossotti suggested the CDC guidelines might be too broad and should instead consider condomless sex acts as the prescribing criterion. Download AVAC’s Advocacte’s Guide to Doxycycline to Prevent Bacterial STIs.
HPV Vaccination and Global Inequities
Namwa Wongkalasin of Imperial College delivered an impressive presentation on the potential of HPV vaccination to prevent non-cervical cancers (i.e., oral and anal cancers), while highlighting the grave challenge of global inequities in vaccine access and coverage—particularly in regions that would benefit most. “Due to unprecedented cuts in global aid funding, this disparity will increase further,” she warned. We are likely to see these effects immediately amid troubling developments at NIH, where grants focused on studying vaccine hesitancy, acceptance, and uptake—particularly those examining HPV and gonorrhea vaccine attitudes—have been terminated. These NIH-funded studies have been pivotal in shaping strategies to boost HPV vaccination rates.
Community Breakfast Club Sessions
Catch up on our CROI 2025 Community Breakfast Club recordings! These virtual sessions, hosted by the CROI Community Liaison Subcommittee, the European AIDS Treatment Group, AVAC, and partners featured researchers and advocates discussing cutting-edge advances in HIV cure, reflections on 40 years of the epidemic, and insights on living long-term with HIV. Watch the recordings now!
And be sure to check out Aidsmap’s news bulletins and in-depth coverage of CROI.
Scientists and advocates will undoubtedly continue to be confronted with the new administration’s assaults on scientific integrity. Read more about how these attacks are expected to devastate HIV prevention, including the capacity to deliver existing PrEP options, scale up new PrEP products, and paralyze the impact of innovative and exciting research like we’ve just heard this week in our special issue of PxWire.