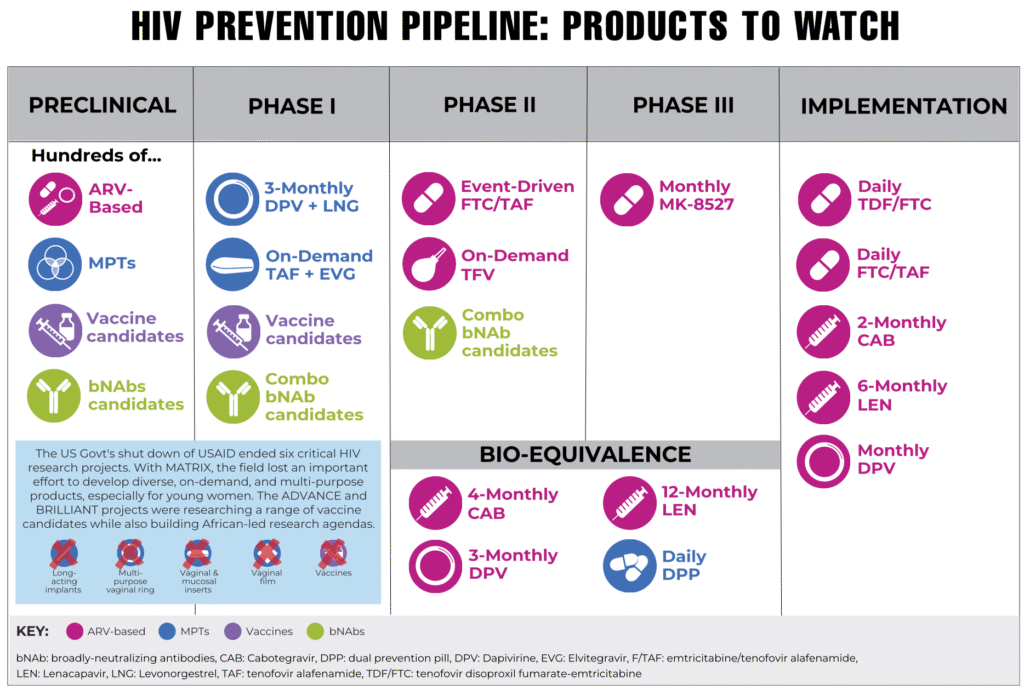
Diminished funding for research and foreign assistance requires sharper priorities and smarter investments to develop and deliver a wide range of products that meet diverse needs. Given the new reality of the HIV response, AVAC and partners also recognize that the conversation around choice must be redefined, and research pipelines must be prioritized and optimized accordingly.
In 2025, AVAC released an updated People’s Research Agenda (PRA) to reflect this context. Created in 2024 with global advocates and communities, the PRA provides a people-centered framework for equitable and accelerated R&D and product introduction. Dr. Jeanne Marrazzo, then-Director of the National Institute of Allergy and Infectious Disease (NIAID), endorsed the PRA at its 2024 launch, coining it a “manifesto for putting community voices front and center.” The 2025 PRA update reflects a critical review of the HIV prevention research pipeline, especially given shifting US political support, emphasizing a strict balance of R&D that is optimized for maximum impact on the epidemic. Its interactive features, including a dashboard for tracking and advocating for HIV prevention R&D, a real-time clinical trials tracker and an action agenda, make it a core tool for advocates, researchers and funders in the current landscape.
The People’s Research Agenda high-level look at the HIV prevention pipeline.
As a trusted ally to community and researchers alike, AVAC also played a leading role in advancing Good Participatory Practice (GPP) in the research program for developing monthly oral MK-8527 PrEP, with support from the Gates Foundation. AVAC engaged nearly 100 advocates from around the world through various engagement platforms and consultations to ensure that trial design, conduct and access plans were designed with community input. As the efficacy program launched, advocates praised the program’s commitment to community engagement and the exciting opportunity MK-8527 offers for expanding choice.
A map of EXPrESSIVE trial sites—a phase III trial of MK-8527, Merck’s monthly PrEP pill.
Similarly, AVAC continued engagement and advocacy in the HIV cure research field. As the community engagement partner to three Martin Delaney Collaboratories and the African HIV Cure Consortium, AVAC drove engagement at local, national and global levels. Through partnerships with African-based civil society groups, cure research was prioritized in country strategic plans in both Malawi and Uganda—a significant win, given the increased need for country commitments to national HIV responses in the
face of diminished US support. AVAC also expanded a global network of cure advocates by replicating the African-focused Cure Advocacy Academies (implemented in partnership with IAS) in the US with the first ever US Cure Advocacy Academy.
Throughout 2025, AVAC continued to be a critical resource for the field, disseminating infographics, quarterly PxWire updates and tools to help partners stay abreast of the evolving research pipeline and introduction status of oral PrEP, the DVR, CAB and LEN. From international conferences to community meetings, AVAC resources were cited widely, affirming AVAC as a go-to source for accurate HIV prevention information and a catalyst for strong advocacy.