Hormonal Contraception and HIV Risk: Understanding the ECHO trial
The Evidence for Contraceptive Options and HIV Outcomes (ECHO) Study is an open-label, randomized, clinical trial comparing three highly effective, reversible methods of contraception — the progestogen-only injectable depot-medroxyprogesterone acetate (DMPA), a levonorgestrel implant, and the non-hormonal copper intrauterine device — to evaluate whether there is any difference in the risk of acquiring HIV infection among users of these methods.
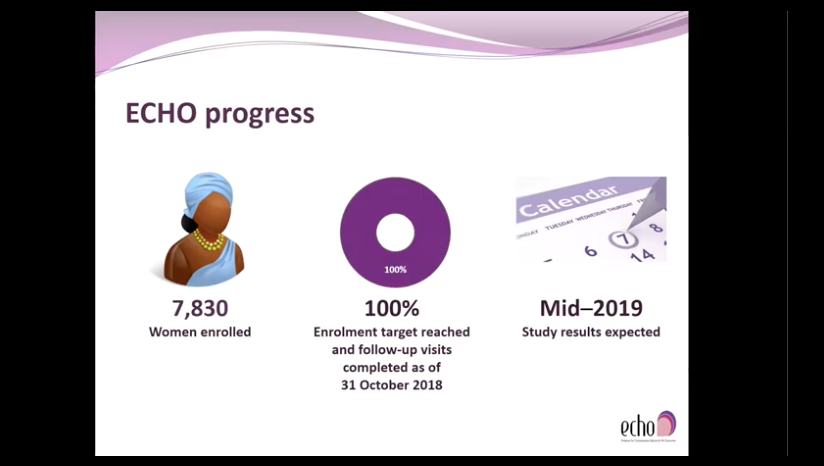
Results, expected in mid-2019, will help guide the implementation of safe, effective policies and services that will enable women at high risk of HIV to make fully informed choices about contraception and HIV prevention.
The webinar featured:
Beth Schlachter, Executive Director, FP2020
Dr. Jared Baeten, Vice Chair, Department of Global Health, University of Washington, ECHO Consortium
Dr. Nelly Mugo, Research Associate Professor, Global Health, University of Washington, ECHO Management Committee
Tamar Abrams, Communications Director, FP2020
You may view the webinar here.
Was this content helpful?
Tell us how we can improve the content.
Was this content helpful?
Thank you for your feedback!