February 21, 2019
Jeanne Baron is AVAC’s web editor and producer of Px Pulse. Manju Chatani-Gada is AVAC’s Director of Partnerships & Capacity Strengthening.
Incorporating stakeholder engagement early and deeply in the development of a clinical trial for HIV prevention research is for us a clarion call. Including a diverse cross-section of expertise and perspectives at the outset, especially of those who may rely on these strategies to prevent HIV, helps research stay on track to eventually deliver options people will trust and use.
A regional gathering in Johannesburg to discuss the design of a trial called MTN 042 showcases several elements that make early stakeholder consultation a powerful tool when so many other meetings under the name of stakeholder engagement are perfunctory occasions.

Also known as DELIVER, MTN-042 will be conducted by the Microbicides Trial Network (MTN) which is funded by the US National Institutes of Health. This study is one of a handful stepping in to fill a vacuum that exists around HIV primary prevention for pregnant women. Data suggest that women are much more likely to become infected during pregnancy and even more likely in the postpartum period. For many women, this represents a significant proportion of their reproductive years. Despite this heightened risk, research often excludes pregnant women in order to protect the developing fetus from possible adverse effects. But this leads to a dearth of data on safe and effective biomedical HIV prevention, such as PrEP, during pregnancy.
MTN 042 will investigate the safety of oral PrEP and the dapivirine ring as HIV prevention for pregnant women. It will also provide information on what kind of trial design will be both acceptable and produce useful results for studies involving pregnant women.
For almost a decade AVAC and the MTN have collaborated on stakeholder consultations, over the years refining our approach based on feedback from hundreds of participants. This work represents a vital aspect to the larger work of Good Participatory Practice, which we encourage readers to learn more about here. The Stakeholders Consultations on MTN-042 in 2018 reflect our best practices in consultations to date.
Manju Chatani-Gada, co-convener of the consultation explains what happened in Johannesburg and why following practices and principles like these mean studies like MTN-042 are better set up for success.
Don’t Confuse an Update with a Consultation
“We often hear of large stakeholder meetings, held right before a trial starts. These meetings can be an important platform to explain the study’s goals, timeline and details about the intervention under investigation, but the meeting participants are not being consulted. Participant feedback may be interesting but will not be used to amend the trial. This is an update,” says Chatani-Gada. In contrast, a consultation poses questions, creates the conditions for an exchange of knowledge, and uses a transparent process for applying the findings to the study’s ultimate design.
At the AVAC/MTN stakeholder consultation, “The [MTN] team made clear what they were taking from this consultation into their protocol meeting, which immediately followed the consultation” says Chatani-Gada.
The agenda of the subsequent meeting to refine the MTN-042 protocol was shaped around issues and recommendations raised at the stakeholder consultation. As the consultation report describes, the next version of the protocol incorporated several of the suggestions made at the consultation. “Not everybody does that. Things are changing but civil society stakeholders have sometimes felt that they have been invited to rubberstamp fully developed plans.”
Get Stakeholders to the Table Early
The consultative process should start with the development of the protocol, which spells out the rationale, objectives, methods and other details of a clinical research study. The protocol should reflect insights that have been gathered from an early and comprehensive discussion with key stakeholders. Ideally, community members should also be part of protocol committees themselves.
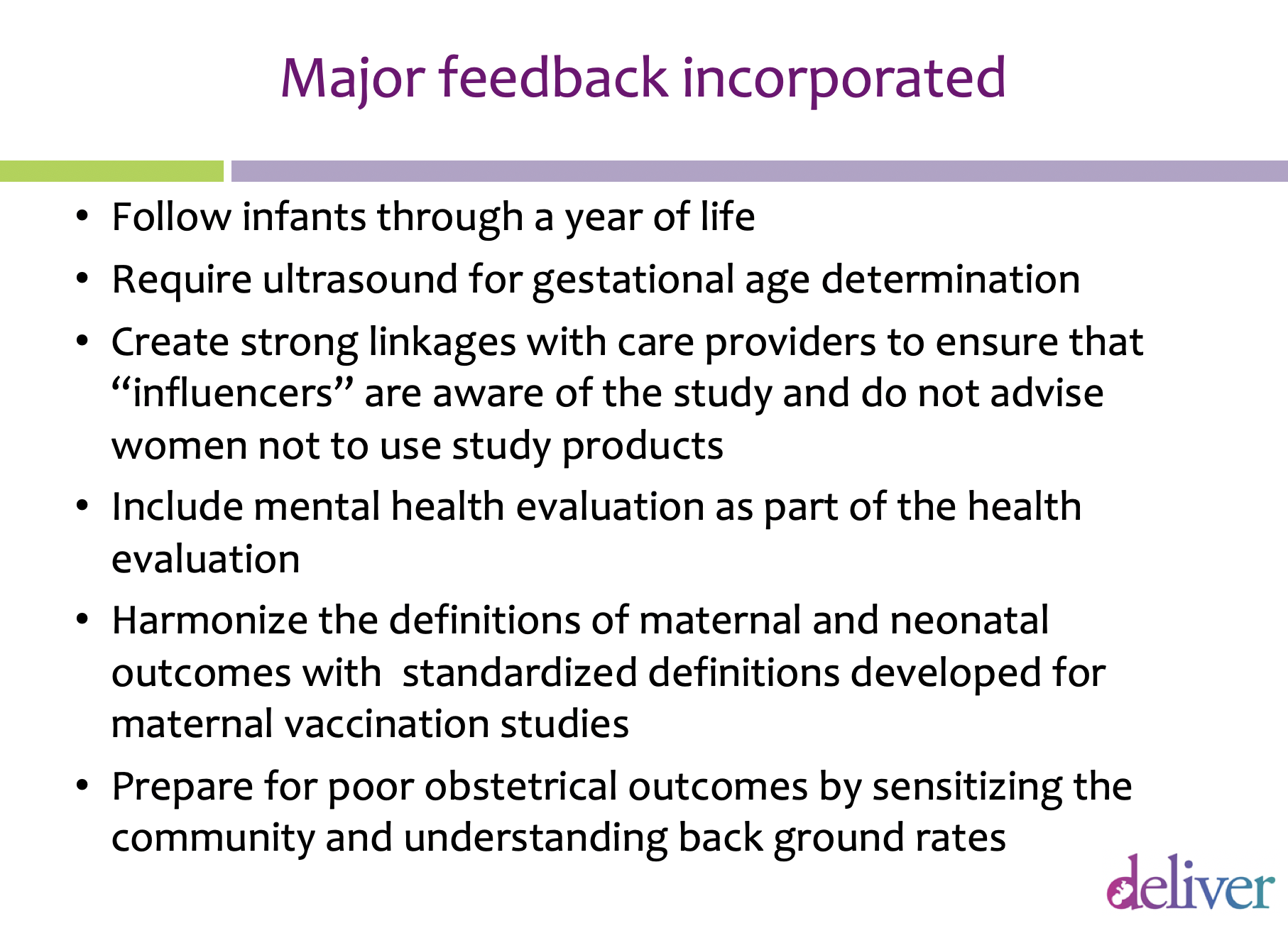
“At this meeting the investigators gained insights about how to shape fundamental aspects of the study so that people will feel comfortable supporting it,” says Chatani-Gada. For example, stakeholders encouraged researchers to follow up with infants born during or after the study for a longer period, and to find ways to establish a better baseline for adverse and healthy outcomes in pregnancy in the relevant communities. In addition to this early regional consultation, there are important country-level consultations. Those will follow in 2019 in Malawi, South Africa, Uganda and Zimbabwe and will include a larger number of women from the proposed trial communities as participants.
Include the Right People and Spotlight Community Voices
It’s critical that the leadership of a study understand the experiences and concerns of potential trial participants and their immediate and broader communities. Relying only on community advisory boards to filter this information may obscure pressures that could complicate the progress of the trial down the road.
“Trial participants, like all people, have complex lives and sometimes face challenging conditions as they make decisions. Researchers need to hear directly from them, and a diversity of other stakeholders too, to better understand those challenges and respond accordingly. Research must fit into the lives of the people in the trial rather than have them fit their lives to research,” says Chatani-Gada.
The researchers behind MTN-042 saw this first hand at the Johannesburg regional consultation. They needed to know if research involving pregnant women could even get through a review process in the places where the proposed trials would take place: Malawi, South Africa, Uganda and Zimbabwe. To explore this question, 35 people were invited to the discussion: researchers; regulators; representatives from ethics committees, ministries of health and the WHO; advocates with expertise on HIV prevention and women’s health and empowerment; and two women from one of the trial-site communities. Both had recently been in a study investigating PrEP use and safer conception. (In these earlier studies, women who became pregnant exited the study. MTN-042 will exclusively study PrEP among women who are already pregnant.)
As people in the room began to share a consensus about moving forward, these two women offered unique and powerful perspectives. Their participation in those earlier trials meant they were relatively knowledgeable about clinical trials and also concerned about their HIV risk. When they were asked, “Would you continue to take PrEP during your pregnancy if you were offered it?” their answers differed. One said, “No, I would not be comfortable joining this trial until I knew the drug was safe.” The other said she would have taken it because she is worried about her exposure to HIV and what it would mean to her child if she got infected.
“This was a gut check and a reminder not to make assumptions about what decisions people will ultimately make. A minister of health, a researcher, a member of the ethics committee is not going to be asked to join a trial. Researchers need to confront these truths and recognize different women will make different choices. Women are not homogenous; their needs are diverse. We can’t presume we know what they need or want, ” says Chatanti-Gada.
Put Everyone in the Room on Equal Footing
To create the conditions for open dialogue, the facilitators set the tone early on in the meeting. “We started the consultation with civil society partners talking about what HIV prevention means to them. So people can get quite personal about how HIV prevention comes up in their lives.This opener grounded the discussion in real-world considerations,” says Chatani-Gada.
“We set the tone by encouraging an informal setting. We relied on first names. There was no Minister So-and-So, or Dr. So-and-So. Everyone participating has to see each other as equally credible and influential, and techniques such as these foster those values.”
It’s also important that everyone involved can engage with the science being presented. “As we often do, we convened a pre-meeting for civil society and community participants in the days before the consultation. Often, we exclude researchers from these sessions. It’s a safe space to break down research terms, wade into the content, and raise questions and concerns. Participants from the pre-meetings routinely tell us these pre-meetings build their confidence in discussing research. It equips them to dig into the material and direct the conversation.”
Encourage Discussion and Questions with New Meeting Practices
It’s important to use multiple methodologies to engage the participants, such as presentations, testimonials, panel discussions, real-time surveys and such. MTN often uses a digital instant-response system to probe important questions. Participants see a question on a screen and can anonymously select from multiple-choice answers. Everyone can see the results in real-time.
“Very interesting conversations flow from these sessions, and it gives the investigators a chance to explore issues that still concern stakeholders.” It also gives researchers immediate answers to the most critical questions they have about a protocol.
For example, this process revealed that almost all participants wanted changes to the makeup of an interim review panel. They suggested African experts be selected over American ones with similar credentials, and a greater number of community representatives should be on it as should experts on pharmacokinetics, drug interactions and certain regulatory matters.
Chatani-Gada also says this set of questions and answers represents concrete findings from the consultation—an overview of what the investigators learned from the participants, which makes transparent how the consultation findings will be carried forward for further consideration.
Commit to Ongoing Stakeholder Engagement
One-off meetings achieve little of lasting value. Instead, broad stakeholder meetings and community-level engagement must be ongoing. They are all critical for research literacy, awareness of HIV prevention and recruitment for the trials. A strong partnership between the research field and civil society relies on continuing dialogue with many different ways to offer feedback.
Follow up is Essential
For example, local organizations will work in partnership with AVAC and MTN to co-convene country-level meetings on MTN-042. Their leadership at the very beginning of the planning stages mean they will help shape the agenda and participant list in the national and local consultations to come, and increase interest. It also means these local partners will play an ongoing role in garnering support for the study’s success. “By working together, the community, civil society and the researchers carry the field forward together. And that’s what we need for HIV prevention research to succeed.”