Continued investment in HIV prevention research critical for keeping the HIV response on track in the coming decade
Contact
Kay Marshall, +1 (347) 249-6375, [email protected]
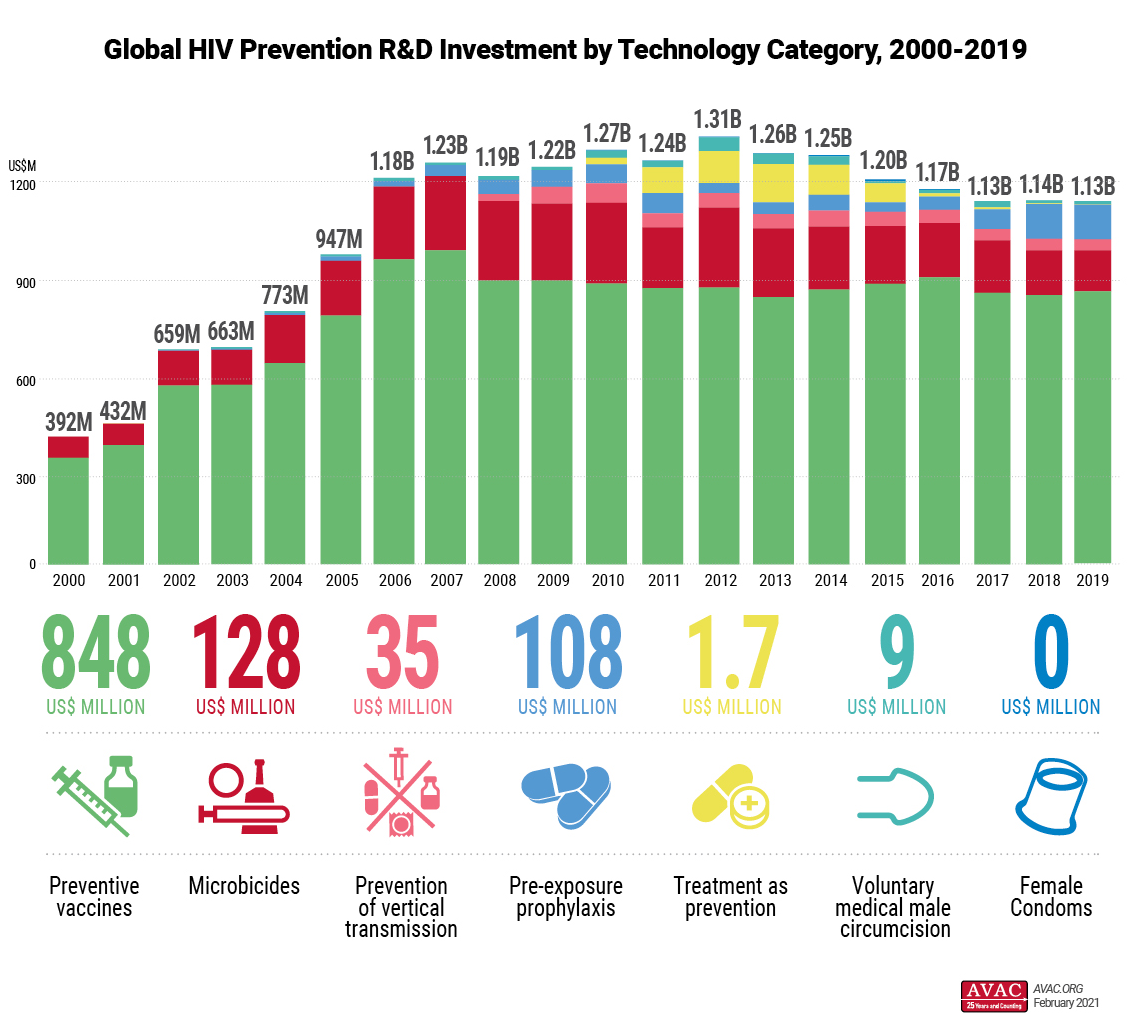
New York, February, 4, 2021 – Funding for HIV prevention research and development (R&D) in 2019 followed familiar patterns seen for almost a decade – overall funding declined slightly in 2019, reversing a slight upward trend seen in 2018 and continuing the overall trend of flat or declining funding since 2012. Total funding for HIV prevention R&D in 2019 was US$1.13 billion according to a new analysis from The Resource Tracking for HIV Prevention R&D Working Group highlighted today at the HIV Research for Prevention Conference (HIVR4P Virtual).
This analysis comes at a time when the field has delivered important clinical trial results that will in coming years broaden the range of HIV prevention options for many people and help the field determine critical next steps in research, development and delivery. Decades of funding for HIV prevention research also helped jumpstart research for COVID-19 interventions, including vaccines and antibody research.
The report found that funding increased for preventive HIV vaccines and female condoms in 2019, while investment in all other evaluated technology categories declined from the previous year. A detailed breakdown of investment by technology category is available on a newly launched website that provides detailed breakdowns of investment accompanied by graphics.
As in the past, public funding made up the majority of funding at US$902 million (80 percent of total funding) and 95 percent of that funding came from the US government. US government funding increased in 2019, with a notable ¬¬increase of five percent from the US National Institutes of Health. Funding in almost every other category, including from European governments, global philanthropies and industry, declined.
New HIV prevention options are moving from labs and clinics toward people’s lives and exciting new products are moving forward in the research pipeline. Decades of sustained funding for HIV prevention R&D has made this possible, although the pace of research has often been slowed by falling or static funding.
It is unclear what impact the turn to COVID-19 R&D support by many of the key funders of HIV prevention R&D will have on funding in 2020 and beyond, but the Working Group warned that funders must continue to support HIV prevention to build on the important gains being made in current research.
The Working Group also noted the running start HIV vaccine R&D gave to the COVID vaccine enterprise. The three-decades-long US$15 billion-dollar-plus quest for a preventive HIV vaccine laid the groundwork for COVID-19 vaccine R&D through advances in computational vaccinology, genetic and vector-based vaccine platforms, antibody assays, viral imaging, clinical trial site infrastructure and other innovations that were successfully repurposed to develop safe and effective COVID-19 vaccines in record time.
Conversely, cutting-edge platforms and technologies developed to combat the COVID-19 pandemic offer new tools and promise in the quest for vaccines against HIV and other infectious diseases, but research to repurpose these advances for HIV R&D must be adequately financed now.
“Even as unprecedented amounts of funding and support continues to flow into the COVID-19 pandemic response, funders must continue – and increase – funding for HIV prevention research and development. Responding to this new pandemic must not take resources away from the ongoing HIV pandemic or the world will see an even greater public health crisis. We’ve learned from COVID-19 that unprecedented funding and cooperation among governments, industry and research groups can speed development of new technologies. Just as COVID research benefited from long-term HIV research investments, lessons learned most recently in COVID vaccine development must now urgently be applied back to HIV prevention R&D,” said Mitchell Warren, AVAC executive director.
“The COVID-19 response has given us incredibly valuable insights and approaches for accelerating the development vaccines and monoclonal antibodies—the foundations of which were significantly enabled by decades of HIV vaccine research innovation,” said Mark Feinberg, President and CEO of IAVI. “As we’ve seen throughout HIVR4P Virtual, the current HIV prevention research pipeline is rich, and the technologies and collaborations that facilitated the rapid discovery and development of efficacious COVID-19 vaccines and therapeutics are already being applied in efforts to advance the development of HIV vaccines and broadly neutralizing antibodies. To build on decades of research progress, we must sustain cross-sectoral investment and collaboration and dedicate increased focus on partnership innovation – which will be as critical as scientific innovation in hastening the end of the HIV pandemic.”
“COVID 19 response has demonstrated that focused efforts and investments can result in the roll out of multiple preventive options against SARS-COV-2 within a record time. Addressing HIV response needs similar efforts and urgent scale-up of investments for HIV interventions and research to end the epidemic.” said Shannon Hader, Deputy Executive Director, Programme at UNAIDS.
The Resource Tracking for HIV Prevention R&D Working Group has employed a standardized methodology since 2004 to generate comprehensive statistics on investment in HIV prevention research and development. Investment estimates that allow comparison across years, prevention options, sectors, and countries provides greater transparency for funders and helps assess the trajectory and impact of policies and funding decisions. The trends documented by the Working Group help predict future funding scenarios that can impact the progress of the historic scientific agenda to find new prevention options to help end the HIV pandemic.
###
About the working group: Since 2000, the Resource Tracking for HIV Prevention R&D Working Group has employed a comprehensive methodology to track trends in research and development (R&D) investments and expenditures for biomedical HIV prevention options. AVAC leads the secretariat of the Working Group, that also includes the International AIDS Vaccine Initiative (IAVI) and the Joint United Nations Programme on HIV/AIDS (UNAIDS). This year’s report is additionally made possible by the support of several donors, including the Bill & Melinda Gates Foundation and the American people through the US President’s Emergency Plan for AIDS Relief (PEPFAR) and the US Agency for International Development (USAID). The contents are the responsibility of AVAC and the Working Group and do not necessarily reflect the views of PEPFAR, USAID or the United States Government.