Last week the Conference on Retroviruses and Opportunistic Infections (CROI) took place online—as the conference went virtual for the second year in a row. There are a number of excellent sources of news to catch all that was shared at the meeting including coverage from aidsmap, HIV-iBase and NATAP. And for recordings of the daily advocate/researcher breakfast updates (or margarita, depending on your time zone or personal preferences) are available at www.avac.org/croi2021. And, of course, there’s AVAC’s take on the news from CROI in a new blog post below. Enjoy!
The Personal is Planetary: CROI and COVID one year on
By Emily Bass
________________________________________
Last week, CROI went virtual as the world marked a year since the World Health Organization officially declared COVID-19 a global pandemic. Like everyone else, I had my own personal milestones from that week last year. I remember a dear friend who’d traveled from Uganda for CROI 2020 sitting in my living room, worrying about whether to attend the event before it pivoted to virtual at the last minute. I remember her deciding to leave and embracing her—unmasked—on the sidewalk in front of my house when she did. I do not have any idea now when we will see each other again.
A pandemic happens to a planet, but it is measured in individuals. Holding both of those realities at the same time is the challenge, and at this year’s CROI, no one did it better than Fatima Hassan and Gregg Gonsalves who shared a screen and billing, as joint presenters of the esteemed Martin Delaney Lecture. The pair delivered it not as a lecture but a conversation, thinking aloud about pernicious manifestations of vaccine nationalism and the inadequacy of current purchasing arrangements to circumvent a level of medical apartheid in vaccine distribution not seen since the era in which they fought for universal access to antiretrovirals. The conversation was simultaneously intimate and impassioned, which may have been why a comment Hassan made in passing nearly moved me to tears. She said she didn’t expect to see Gregg, a friend for two decades, “for years.”
Hassan and Gonsalves said many critical things in their talk—which CROI has made available (an exception to a new and regrettable policy that all 2021 content will remain behind a paywall for six months). Their clear, methodical and damning conversation tied today’s pandemic to the early years of HIV/AIDS. Gonsalves quoted irascible, irreplaceable AIDS activist Larry Kramer who said that HIV/AIDS was about “disposable people”, the Black, brown, queer, immigrant and poor people who are not valued by the capitalist state. “Now we see [with COVID] another set of disposable people,” Gonsalves said. “Many of us didn’t think we would have to see this again.”
Immediately after their talk, a working group came together to draft and launch a Call for Vaccine Equity and it was subsequently published in BMJ. It outlined a set of demands including a call for the United States government to incentivize pharmaceutical companies like Pfizer, Moderna and Johnson & Johnson to share knowledge on how to produce their vaccines; World Trade Organization members to waive the trade-related intellectual property (TRIPS) provisions that serve as a barrier to generic production; and COVAX partners to acknowledge that its anticipated vaccine coverage rates of 20 percent of the population of countries dependent on COVAX facilities is simply too low. The cost of inaction will be measured in numbers that sometimes make the mind go numb; it will also be measured in the time that comrades spend separated by continents but bound in the common fight for equity.
This is not a test: Time to tackle HIV diagnostics, counseling messages, pricing and more for PrEP in its many forms
CROI brought new information on long-acting injectable cabotegravir (CAB-LA) for PrEP, which has previously shown high levels of efficacy in reducing risk of HIV in gay men and other men who have sex with men and transgender people who have sex with men in a trial called HPTN 083; and in cisgender women, in a trial called HPTN 084.
Building on data presented at AIDS 2020, Dr. Raphy Landovitz presented an in-depth analysis of the timing of HIV infection in HPTN 083 participants, relative to when they were diagnosed with HIV, what product they were receiving, and how much of the drug was present in their blood.
A highly-detailed summary of the findings can be found here, in Gus Cairns’ always-excellent coverage for aidsmap. Broadly speaking, there are three key findings that advocates will need to react to and act on as part of a comprehensive PrEP agenda:
1. In people using injectable CAB-LA and daily oral TDF/FTC PrEP, standard HIV tests do not always provide accurate, real-time results. Most HIV tests look for antibodies to the virus and/or antigen (a molecule found on the surface of the virus) from HIV. People who have very low levels of HIV, or who have acquired HIV very recently, do not always have antibodies to the virus—and so they will test “negative” on standard diagnostics. Taking an antiretroviral like CAB-LA can suppress HIV to very low levels for a time, meaning that people who acquire HIV while receiving the injection may not test positive on standard diagnostics.
This was the case for four participants in HPTN 083 who acquired HIV even though they had received shots of CAB-LA as scheduled. These individuals did, eventually, test positive on the standard HIV antibody and antigen tests used at study visits, but when the trial team looked back at blood samples from these four people at prior visits, and used more sensitive laboratory diagnostics, they found that each had acquired HIV weeks, if not months, prior to the first positive test result at the site. Landovitz said there is “a need to look [for HIV] with diagnostics that are appropriately sensitive. And with long-acting agents for prevention [e.g., cabotegravir], currently available diagnostics are blunted or delayed in sensitivity.”
A presentation by Dr. Donn Colby looking at people using daily oral PrEP in Thailand also found eight individuals who had HIV at the time that they initiated oral PrEP but tested negative on standard HIV tests at enrollment. Five of these individuals tested were identified via a pooled RNA test (a group of samples tested for signs of HIV genetic material, which is detectable earlier after infection than antigen or antibody). Three individuals tested positive after starting PrEP. As in HPTN 083, the site went back to look at previously collected samples and found viral RNA when an array of standard tests gave a “negative” result.
The number of people with HIV whose virus isn’t picked up via standard tests is relatively small. Colby estimated about one case of acute infection (the term for the very early phase after HIV acquisition) per 400 people initiating PrEP at the Thai PrEP clinic; in HPTN 083, the four individuals with HIV at baseline (described above) were out of a group of 2,282—which is one per 570.
But even with these small numbers, there are big questions: how should the risk of a false negative be conveyed to people when they start a PrEP strategy like CAB-LA where it appears that there can be a long interval between infection and diagnosis via antibody/antigen tests? What approaches can be used in programs when a person is starting PrEP initiation? Finding people with acute HIV infection allows them to start treatment right away if they choose to do so. It also minimizes the risk that a person with HIV will start single-drug PrEP, which can lead to drug resistance.
Both of the solutions shared at CROI are practical in some ways and present challenges in other ways. In the Thai clinic, people who report a behavior in the past month that is associated with high risk of HIV are initiated on three-drug ART for the first month of PrEP, before a confirmatory negative test allows them to shift from three-drug ART to a single drug for PrEP. Both Colby and Landovitz talked about the need to consider using viral load assays to confirm HIV status. These tests, used to detect HIV in the blood of people living with the virus, are far more sensitive than diagnostics; they’re also far more expensive. Hearing this suggestion, in the context of ongoing gaps in coverage of viral load testing for people living with HIV, immediately made me think of the conversation around oral PrEP in the years following the first evidence of its efficacy. Then, treatment waitlists and shortages of TDF/FTC for people living with HIV made a push for expanded rollout of TDF/FTC for prevention untenable for many activists. Calls to introduce viral load testing as a diagnostic for either CAB-LA, oral PrEP or the Dapivirine Vaginal Ring must happen in the context of a concerted, funded effort to provide universal viral load coverage—at least one viral load test per year—to all people living with HIV. This is by no means a given.
CROI itself featured presentations on approaches that could provide cheap, early alternatives to viral load tests as means of determining when a person’s current HIV drug combination is no longer suppressing her virus. (A detectable viral load is used as a sign of “treatment failure.”) Dr. Jose Castillo-Mancilla presented on the use of dried blood spot tests to detect emtricitabine triphosphate. This form of TDF/FTC is only detectable in the blood within 36 hours after a person has taken a pill; testing for presence or absence is a way of finding out whether a person has recently missed a dose. Castillo-Mancilla and his colleagues found that people with no detectable emtricitabine triphosphate were more likely to have detectable viral loads in the future. Dr. Jacantha Odayar and her colleagues in a South Africa study found that presence or absence of tenofovir diphosphate (another form TDF/FTC takes as it is broken down, or metabolized, in the body) in blood predicted future viremia in pregnant women. Such tests, which focus on TDF/FTC containing regimens, could be used as a “early warning” system for predicting virologic failure among people living with HIV.
2. Counseling needs to catch up to high-tech PrEP. Landovitz’s presentation also suggested that rolling out CAB-LA for PrEP will require counseling strategies for people who test positive while receiving the injections as prescribed. In HPTN 083, one of the people who acquired HIV, despite on-time injections, initially refused to accept the diagnosis in part because standard test results were inconclusive. This individual opted for a month of post-exposure prophylaxis instead of starting treatment. Delays in diagnosis, confounding results on standard diagnostics and individual disbelief in positive test results will all come up in “real-world” use. Counseling and follow-up must be developed with these needs in mind.
A presentation by Dr. Catherine Koss from the SEARCH study offered another reminder that social networks play a key role in PrEP use. The study made a valuable investment in looking at both community, social and structural aspects impacting health behaviors. SEARCH used an elaborate system of contact mapping and concluded that people who knew someone using PrEP were 57 percent more likely to use PrEP themselves. Destigmatizing and normalizing PrEP so that people talk about it when they choose to use it (or stop or restart it), and incorporating peer-to-peer support for all forms of PrEP into programs will likely be key.
Resistance happens among people taking CAB-LA but not necessarily during the “tail”. Long-acting products like CAB-LA include a period, after a person has stopped receiving injections, when the drug is still in the body but at levels that don’t provide protection against infection—often referred to as the “tail”. (For any PrEP strategy, there’s a blood-drug level that’s associated with prevention; you don’t need “some” PrEP drug but “enough” to reduce HIV risk.) There’s been real concern about drug resistance arising in people who acquire HIV during the tail. But in the three people who fell into this category in HPTN 083, none had HIV with genetic mutations that render it resistant to integrase inhibitors, the class of drug that includes cabotegravir. This number is too small to support conclusions about the risk of resistance, but in this handful of people, resistance didn’t emerge during the tail. When integrase inhibitor resistance did emerge in HPTN 083 participants, it was in people who were infected and also receiving the injections: one of the people who had HIV at the time of enrollment developed resistance, as did two people who acquired HIV during the oral lead-in phase (a trial period where individuals took the pill form of CAB-LA before receiving the injection, to ensure that the drug was well-tolerated). Two people who acquired HIV while receiving CAB-LA injections also developed integrase inhibitor resistance; two others had so little virus in their blood that the team could not get a resistance test. The take-home message—at least from this trial—is that the risk of resistance is greater when people acquire HIV while taking CAB-LA as prescribed than it is in people who’ve stopped receiving the injection, i.e., in the context of the “tail”. Cabotegravir is an integrase inhibitor, as is dolutegravir (DTG), which is used as first-line treatment in many settings. The people with integrase-inhibitor-resistant virus responded to alternative regimens, including TDF/FTC-efavirenz-based therapy and a regimen using a boosted protease inhibitor. In resource-constrained settings where countries have fully embraced DTG-based first-line therapy, it will be essential to plan for CAB-LA introduction in the context of treatment options for those with integrase inhibitor-resistance.
3. Injectable PrEP is “superior” to oral PrEP among people who can’t or don’t adhere to the daily pill. Landovitz also presented an analysis of serum and dried blood spot samples from HPTN 083 participants who were assigned to receive daily oral TDF/FTC, the comparison arm of the study. (In each arm, people also received a dummy, or “inert” version of the other product—people randomized to TDF/FTC received saline injections, people receiving CAB-LA injections received dummy pills.) Thirty-seven of the 39 individuals who were prescribed and counseled on the active daily pill and went on to acquire HIV had “suboptimal or non-adherent” levels of the drug in their samples at the time of infection. The study concluded that CAB-LA was “superior” to oral TDF/FTC. These new data clarify that people who received CAB-LA injections on schedule every two months had a substantially lower risk of HIV than people who received daily oral PrEP but did not or were not able to take it as prescribed. In other words, the difference in incidence rates relates to product use, and not the product itself.
One strategy is not inherently superior to the other, i.e., injections and daily oral PrEP each taken exactly as prescribed may well provide comparable levels of protection; the study didn’t evaluate this question.
Products exist in the real world, where people’s lives, circumstances, bodies and preferences play a role in what’s possible and pleasurable. In the real world—as several CROI presentations highlighted—starting and staying on daily oral PrEP is a challenge for some. The 083 data say much the same. “Choice is the answer,” declared the inimitable Dr. Linda-Gail Bekker in her plenary presentation, emphasizing that many oral PrEP programs see people using the strategy with complex patterns of starting and re-initiation. Understanding these patterns of use—and their impact on both individual risk and population incidence—is key to understanding the impact of PrEP.
Time to calculate the cost of progress—and of inaction
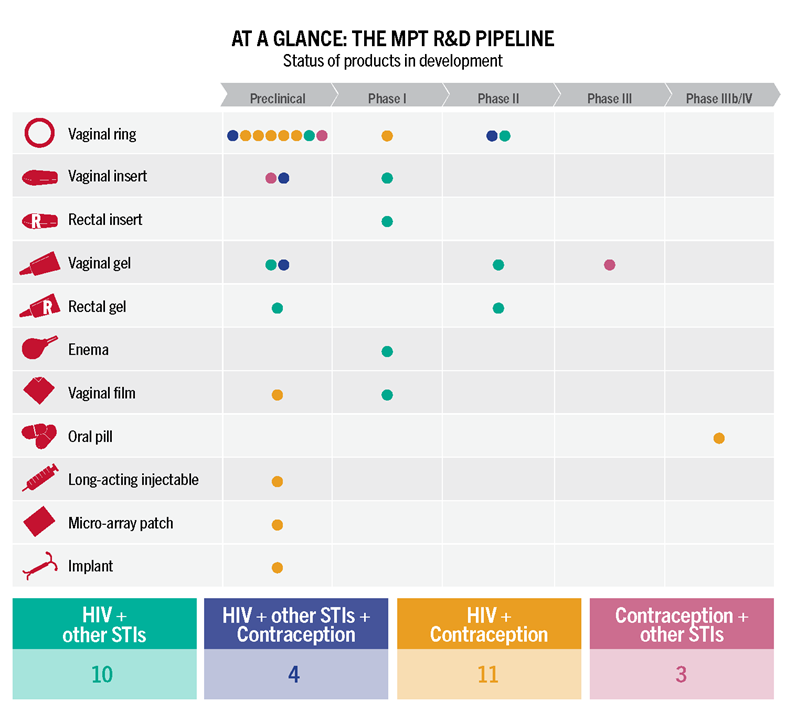
CROI also brought updates from product developers working on other types of long-acting PrEP, including a one-year implant containing the drug islatravir that could be placed under the skin for continuous protection, and a Dapivirine Vaginal Ring (DVR) that could be worn for three months. This is three times as long as a monthly ring that has received a favorable opinion from the European Medicines Agency and been recommended by the WHO. The International Partnership for Microbicides is presently submitting the monthly DVR for regulatory approval in a range of countries.
Islatravir (both as an annual implant and as a monthly pill, which is now in efficacy trials), a three-month ring and the six-monthly injectable lenacapavir look promising and would make choice-based programming real by expanding options and allowing prevention programs to meet people where they are. Long-acting PrEP might, for example, play a role in HIV prevention for postpartum or breastfeeding women. A study of PrEP use among pregnant and breastfeeding women found higher PrEP uptake among pregnant women compared to those who were not pregnant, and a drop-off in PrEP use during breastfeeding. But risk remains high for HIV-negative women who are breastfeeding—a reminder that matching prevention offerings with lifecycle events is a life-saving imperative.
All of these offerings will come at a cost, of course. A “superior” strategy like CAB-LA could reduce HIV in many people, but could also require new testing tools and counseling strategies—and still won’t work for everyone, including transgender individuals with buttock implants, unless an alternate injection site can be identified. A 90-day ring and a one-year implant will, similarly, offer transformative options for reducing HIV risk, but will not address rates of STIs that remain very high in many study populations. Transformative PrEP programs will be ones that offer choice in both HIV and contraceptive options, and sexual and reproductive health care, including STI prevention and treatment, for people of all gender identities and sexual orientations. It’s not possible to plan or budget for such programs without knowing the cost of the products themselves. To date, ViiV, the developer of CAB-LA, has not set a price for CAB-LA. A modeling study presented by Dr. Anne Neilan asked the question, “How much should we be willing to pay for CAB-LA for MSM and transgender women, compared to generic daily oral TDF/FTC, or branded FTC/TAF.” Using a set of prices specific to the Global North, Neilan and her colleagues proposed that the injection would need to be priced comparably to generic daily oral PrEP for it to be considered “cost effective”.
While such models and projections always have limitations, they provoke useful and necessary discussions. Will the price be set according to this calculation? If it is not, will gaps in PrEP access open as they once did with antiretrovirals for HIV, and as they have for COVID-19 vaccines? Or will the HIV field, in all its diversity, set an example for how to learn from history and advance equity for the future?
At the end of CROI, I was no more certain of this than when I might see my Ugandan friend again—or when Hassan and Gonsalves might next sit side by side in real life. But I did know, in my bones, that if there was progress towards equity and justice it would come from the human connections between activists, allies and friends that have always transcended physical distance out of love and anger, and a commitment to lasting change.