Today, May 18th, is HIV Vaccine Awareness Day (HVAD), and advocates are calling for policies, science and advocacy for HIV vaccines that leverage the lessons of the past year.
This HVAD, we are focused on how the COVID experience can speed the development AND delivery of vaccines for HIV, TB, malaria and other diseases. Below you’ll find a roundup of resources, from AVAC and others, providing context, key messages and other information on what the field has learned from COVID-19, the essential role of vaccines in global health, and what’s next in the search for an HIV vaccine.
Vaccines & Global Health in Perspective
- In a commentary today in Bhekisisa, Fatima Hassan of Health Justice Initiative and AVAC’s Mitchell Warren outline Finding an HIV vaccine: Five lessons from the response to COVID-19.
- Episode 1 of the new podcast from the International AIDS Society (IAS), HIV Unmuted takes listeners to the global HIV change-makers who have shaped the response and asks what must happen now to end the AIDS epidemic. This debut episode features the NIH’s Anthony Fauci, AVAC’s Maureen Luba, Udom Likhitwonnawut from Thailand’s National Community Advisory Board, and others.
- In their piece, How HIV Vaccine Advocacy Can Leverage Lessons from COVID-19, USAID’s Ashley Lima and Margaret McCluskey draw on the experiences and perspective of CASPR partners and their agenda for vaccine advocacy in the midst of COVID.
AVAC’s HVAD Package
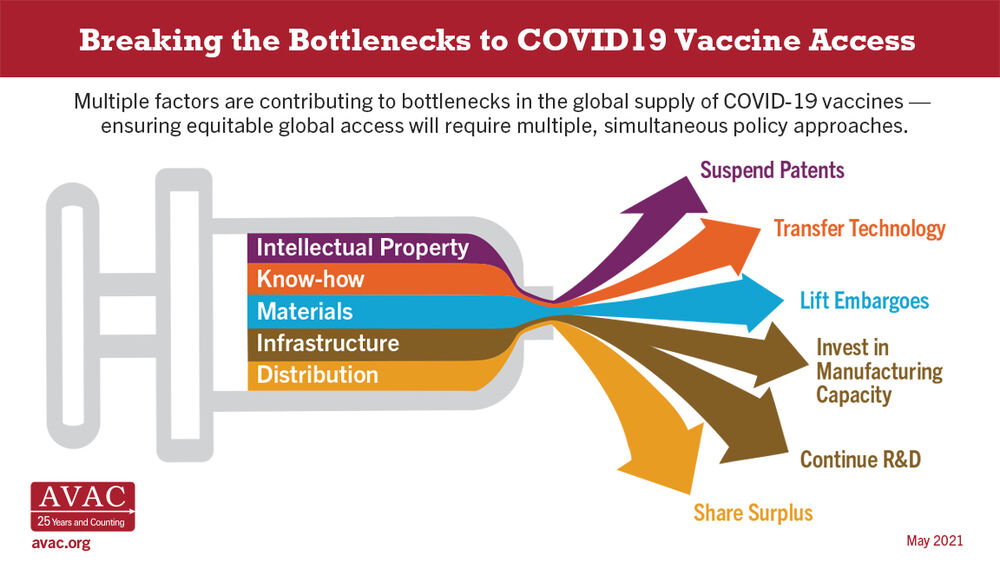
Check out AVAC’s key messages for this HVAD. Designed for easy communication, this resource outlines the six ways that the COVID experience can shape HIV vaccine advocacy moving forward. For social media, we hope our sample tiles and tweets will inspire action to keep audiences #HIVvaccineaware this #HVAD2021. Download tiles 1, 2, 3, 4, 5, 6. Get the basics with AVAC’s annually updated powerpoint tutorial on HIV vaccines; current and planned vaccine research with this table of clinical trials; and our infographics gallery allows you to search by prevention intervention. All of these resources and more are up at www.avac.org/hvad.
IAVI’s HVAD Resources
We encourage you to explore IAVI’s #HVAD2021 toolkit for the perspectives of scientists and community liaison teams, videos on the science and development of vaccines, and tiles and tweets supporting their HVAD campaign at #EyesOnTheTarget and more.
Updates from the Field
- Earlier today, WACI Health and AfNHI hosted a webinar exploring the remarkable advances in HIV vaccine research and the promising concepts being studied today with Ntando Yola, of APHA-South Africa and the Desmond Tutu Health Foundation, and Nyaradzo Mgodi, clinical pathologist from the University of Zimbabwe. Look for a recording of the webinar here, along with recordings of two additional webinars: one co-hosted by IAVI and KANCO featuring leading researchers and advocates in East Africa and one co-hosted by IAVI and SANAC featuring leaders in South Africa.
- On May 13th, AVAC hosted a webinar HIV Vaccines in the Midst of COVID. In this recording you’ll hear researchers and advocates discussing new vaccine technologies, delivery challenges, community engagement, and confidence in both vaccines and vaccine research.
- In the latest episode of AVAC’s Px Pulse podcast, Dive into the AMP Trials, we unpack the results of the recent Antibody-Mediated Prevention (AMP) studies, which will inform the fields of antibodies-for-prevention, and HIV vaccine research.
We can make #HVAD2021 the turning point, when the full potential of vaccines becomes undeniable, and the world renews its commitment to the search for vaccines for HIV, TB and other diseases that continue to threaten so many people’s health and well-being.