There will be no issue next week. The NewsDigest will return on January 7, 2022. Our best wishes for happy holidays and a peaceful new year, and our thanks for reading!
The Weekly NewsDigest will return January 7
Ups & Downs in the Field: Setting an Agenda Together for HIV Prevention in 2022
Join AVAC on Thursday, January 13 at 9:00 am EST for Ups & Downs in the Field: Setting an Agenda Together for HIV Prevention in 2022, a webinar to discuss the latest ups and downs in HIV prevention, and set an advocacy agenda together for the future. Click here to register.
A World In Transition: Charting the Future of HIV Prevention and Global Health Advocacy and Action
We close the year with the exciting news that injectable cabotegravir has gained FDA approval as PrEP. It’s an important achievement, adding another much needed HIV prevention option to the mix. But as we reflect on the many lessons of the year, we recognize this milestone sits within a larger, more complicated context. This regulatory approval is only one, albeit essential, step (for a single product among a much-needed pipeline of many) in a greater effort to transform options into real choices that have impact on epidemics and in people’s lives.
The story of 2021 is one of advances, disappointments, uncertainty, perseverance and solidarity. Through it all, we recognize themes that have been similar throughout AVAC’s 26-year history: follow the science, center community in the response, and lead with equity, always.
2021 brought no end to challenges in global health, and we hope that everyone finds time to reflect, and, hopefully, rejuvenate for the year ahead and the work that we hope to do collaboratively.
To start the new year right, please mark your calendars for Thursday, January 13 at 9:00 am EST for a webinar to discuss the latest ups and downs in HIV prevention, and set an advocacy agenda together for the future: Ups & Downs in the Field: Setting an Agenda Together for HIV Prevention in 2022; click here to register.
In the meantime, if you’re looking to catch-up on the year that was, planning for the year ahead, or just want to have some good holiday reading and listening, here are a few important reminders of 2021:
- On the heels of this week’s FDA approval of injectable PrEP, and with decisions from other regulatory bodies anticipated in early 2022, check out An Advocates’ Primer on Injectable Cabotegravir for PrEP and explore our Biomedical Prevention Implementation Collaborative (BioPIC) that is working to close the gap between research, regulatory approval and rollout for injectable cabotegravir and future products.
- In January, WHO officially recommended the Dapivirine Vaginal Ring (DVR) be included as a prevention choice for women at risk of HIV. The regulatory approval process has taken longer than we all wanted with various twists and turns, but happily WHO just this month restated their commitment to support countries as they consider whether to include the DVR as an additional prevention option for women.
- With the introduction of the new ring and injectable PrEP on the horizon in 2022, it has never been more important to reflect on the lessons we recently documented from the past decade of oral PrEP and their implications for these next generation PrEP products.
- Even as the new PrEP options rollout, the need to develop additional PrEP options continues. We have a full menu of resources related to the research agenda. New ethics guidelines support ongoing and future trials in an era of existing PrEP, which we reviewed earlier this year; our Px Pulse podcast series Research Fundamentals looks at the fundamental role of endpoints in trial design; our continued focus on next generation trial design includes this quick reference to critical updates on ethical guidance in HIV prevention trials; and a new fact sheet on Evolving Designs for HIV Prevention Trials that includes descriptions of new trials of the six-monthly injectable lenacapavir and once-monthly oral islatravir. Unfortunately, news in the past two weeks about holds on both the lenacapavir and islatravir trials (for very different reasons) are important reminders of the uncertainties of product development AND the enduring need for research literacy, ongoing stakeholder engagement and Good Participatory Practices to help navigate it all.
- Beyond ARV-based PrEP, what’s the future of antibody mediated prevention, following important results that were presented in January? Read our Understanding Results of the AMP Trials, and listen to our Px Pulse podcast with a Dive into the AMP Trials.
- Nothing about HIV vaccine research has ever been easy, and results of the Imbokodo study reminded us all both how difficult this research is and how essential it continues to be. Listen to our webinar from September to understand the results and their implications, and be sure to check out this special supplement on HIV vaccine R&D in the Journal of the International AIDS Society, including a number of critical articles from AVAC staff and partners. And if you want to learn even more about how HIV vaccine research paved the way for COVID mRNA vaccines—and how HIV might get paid back—be sure to watch this CNBC video with a number of leading researchers and advocates, including our Executive Director.
- Speaking of COVID-19, AVAC and many partners have spent the past two years developing the COVID Advocates Advisory Board (the CAAB), which is playing a critical role in engaging and convening essential conversations in responding to yet another pandemic. Check out the CAAB’s new website, and listen to a webinar on the global readiness for vaccine manufacturing with New York Times reporter Stephanie Nolen and another remarkable conversation on the Omicron variant with CAPRISA’s Slim Abdool Karim.
- In HIV or COVID or any area of public health, representation matters. It’s core to effective advocacy, and one of the reasons we are so excited about No Data, No More: Manifesto to Align HIV Prevention Research with Trans and Gender Diverse Realities. Developed in collaboration with trans and gender diverse activists from Cape Town to Berlin, this report takes critical steps toward a comprehensive research agenda for HIV prevention that serves trans and gender diverse people.
- Leadership also matters, which is why we are grateful to Acting US Global AIDS Coordinator Angeli Achrekar for her continued efforts to advance PEPFAR and excited about the nomination of John Nkengasong to lead PEPFAR going forward. Read more about our views here and here.
- Last but not least, 2021 got us at AVAC thinking not only about the larger field but our place in it. We undertook the most encompassing reflections on our work since AVAC was founded 26 years ago. As part of this process we reviewed our work from top to bottom and undertook a months-long process to forge a strategy for the years ahead that will advance HIV prevention in the broadest context necessary to advocate for global health equity. We are proud to share with you our strategic plan through 2026 and our commitment to equity, diversity and inclusion. We also issued a preview of our 2021 AVAC Report: Developing Options, Delivering Choices, which describes the concerted actions needed to transform prevention “options”, developed through research, into prevention “choices” that reach the people who need them most.
What a year—and what a “to do” list for our ongoing collaborations with all of you! We look forward to working with you to understand how the field is evolving, where it’s stuck, and what we will do together to advance HIV prevention and drive global health equity forward—so please do register to join us on Thursday, January 13 at 9:00 am EST to discuss the latest ups and downs and set an advocacy agenda together for the future.
AVAC Applauds FDA Approval of Injectable PrEP
Yesterday, December 20th, the US Food and Drug Administration (FDA) issued welcome news. It has approved injectable cabotegravir (CAB-LA, and brand name “Apretude”), the first injectable form of HIV PrEP. As another form of HIV PrEP that does not require taking a daily pill, CAB-LA is a much-needed addition to a proven HIV prevention toolbox that now also includes male and female condoms, daily oral PrEP, voluntary medical male circumcision (VMMC) and the Dapivirine Vaginal Ring.
“The approval of CAB-LA is a welcome and much-needed boost for HIV prevention,” said Mitchell Warren, executive director of AVAC. “With as few as six shots per year, this highly effective form of injectable PrEP can help bend the curve of the HIV epidemic – but only if its approval is accompanied by strategic, effective and equitable rollout that transforms the growing list of HIV prevention options into real and accessible choices for the people who need prevention most.”
CAB-LA is an injectable antiretroviral given to adults and adolescents who are confirmed to be HIV-negative at two-month intervals to reduce the risk of HIV. While today’s FDA action approves CAB-LA for use in the United States only, AVAC and its partners will be working in the months ahead to support the review of CAB-LA by regulatory authorities in other parts of the world where new HIV prevention options are sorely needed. AVAC is heartened that ViiV Healthcare, the developer of CAB-LA, has submitted applications to multiple regulatory authorities, including Brazil and several in sub-Saharan Africa that hosted the pivotal clinical trials led by the NIH-funded HIV Prevention Trials Network (HPTN).
“The experiences of oral PrEP for HIV prevention and from COVID vaccines are stark reminders that the US FDA approval is just one small, albeit important, step in translating exciting science into public health impact,” said Warren. “Without global regulatory approvals, clear guidance from WHO, a commitment to equitable access and fair pricing, and resources to deliver innovation, the best science does not prevent or end pandemics.”
The US approval of CAB-LA is an important and welcome milestone in HIV prevention, however, it is just the first in a series of steps needed to ensure that injectable PrEP can help reduce the 1.5 million new HIV infections that occurred in 2020. Supporting access to injectable PrEP, oral PrEP and the full range of proven prevention options requires programs that are strategically designed, user-centered, appropriately resourced, and promoted and designed to reach those who need prevention most, as outlined in the work of the Biomedical Prevention Implementation Collaborative (BioPIC). Lessons learned from nearly ten years of experience in supporting access to oral PrEP will be particularly important in shaping broad and effective access to injectable PrEP, alongside oral PrEP and the Dapivirine Vaginal Ring, and are detailed at prepwatch.org.
Effective global use of CAB-LA for HIV prevention will also require a significant and long-overdue upgrading of global HIV testing capacity, as injectable PrEP can only be used safely if the recipient is HIV-negative and is tested before every dose. It will also require advocacy around self-testing and lower age of consent to testing policies, which have been significantly correlated with oral PrEP initiations.
“Transparency and fairness in pricing, advocacy to accelerate global regulatory review, feasible testing policies, and upgrades to health systems are crucial to effective access to injectable cabotegravir and must all be part of the global HIV prevention agenda moving forward,” noted Warren. “Today’s approval announcement is warmly welcomed, but is also just the start of efforts to make long-acting injectable PrEP an accessible choice for all in need.”
PrEP’s Time has Come
Early planning for PrEP investment in PEPFAR countries is the next critical step for this intervention to fulfill its promise and bend the prevention curve of the epidemic in PEPFAR supported countries.
The President’s Emergency Plan for AIDS Relief (PEPFAR) has played a fundamental and unique role in bringing down the number of global deaths from AIDS and advancing global health, since it launched in 2003. But when it comes to preventing HIV, not just treating it, the world remains in crisis. Despite important declines in HIV rates in Eastern and Southern Africa, in others HIV is on the rise. A global health target to bring down new cases of HIV to 500,000 in 2020 was missed–by a lot, throwing off the global effort to end the epidemic by 2030 unless drastic action is taken now.
PEPFAR has an unparalleled ability to marshal data and support the development of effective programs, as it has done for treatment in countries hard hit by HIV. Today, PEPFAR must apply this capacity to HIV prevention in new and expanded ways. Some of the most crucial decisions about PEPFAR’s role in delivering prevention are too often overshadowed by the critical center-stage effort to achieve the 2025 treatment targets and reduce unacceptably high AIDS deaths in many countries. But some less known, behind-the-scenes decisions are also truly vital. One such issue involves PEPFAR budget codes that few may appreciate and love but have an outsized impact on whether the right commitments are made, and prevention reaches those who need it most. Currently, PEPFAR utilizes 19 budget codes for specific areas of HIV programming including testing, treatment, and prevention, including voluntary male medical circumcision.
PEPFAR’s budgeting approach to oral PrEP is a prime example of how HIV prevention must evolve and how PEPFAR’s long standing commitment to transparency can help lead the way. Oral PrEP is a daily pill that can be taken to prevent HIV. Despite its outstanding efficacy, not one of the budget codes PEPFAR uses for HIV programming tracks spending on PrEP. PrEP expenditures are only identified after they are allocated during the annual Country Operating Plan (COP) process used to develop individual country plans. Country programs designing prevention programming, and most importantly civil society advocating for PrEP as part of the COP process, don’t have a clear budget line as COP plans develop to measure and advocate against. As a result, PrEP programming, although a priority for PEPFAR, can sometimes get lost in the shuffle of COP planning. A budget code provides a benchmark to plan against and cements a program as a priority for PEPFAR.
The story of voluntary medical male circumcision (VMMC) rollout highlights the difference a dedicated budget can make. After the WHO and UNAIDS recommended VMMC for HIV prevention 2007, PEPFAR dedicated funds for countries to scale it up, and tracked the effort with an individual budget code. VMMC has since become a core component of prevention programs in PEPFAR supported countries. Investments in VMMC, totaling more than $1.5 billion according to 2017 information, are used for equipment, supplies, monitoring, evaluation, training and reporting. Since 2007, VMMC underwent a scale-up of historic proportions, reaching 15 million people, and contributing to lowering incidence in those countries. The budget code entailed more than funding and services, it incorporated planning, tracking, accountability, and impact. As the figure below suggests, the experience of PrEP roll-out now almost a decade after FDA approval without a designated budget code has been much slower.
PEPFAR’s track record in implementing HIV programs is historic. The program is credited with preventing infection in nearly 3 million babies, providing treatment to more than 17 million people who live with HIV, providing testing to 50 million people, and training 300,000 thousand new health workers. To finally end the epidemic, this same capacity can and must be brought to bear in prevention. PEPFAR’s strong call for programs to include oral PrEP and setting an overall goal of serving 1 million people with PrEP in 2021, become more difficult targets to aim at with vital planning tools missing. PEPFAR’s experience with VMMC taught us that establishing a designated budget code signals that a given program or product is a priority. PEPFAR supported countries are readying now to plan for COP22, to begin October 1, 2022. Getting a budget code for PrEP approved for COP22 would be an important step in planning for PrEP programs so that targets are met, and funding can succeed.
Ten years of effort to roll out PrEP has brought powerful lessons. The field has learned about complex barriers that inhibit HIV prevention. Stigma and economic hurdles put HIV prevention, such as PrEP, beyond reach for the millions who need it. Overcoming these forces will depend on critical investments that must be monitored for effectiveness. These include peer-led adherence support, marketing strategies to understand who must be reached and how to reach them, public campaigns to generate demand, reliable supply chains, specialized training for providers, and integrating HIV prevention with sexual and reproductive health services. Funding this full spectrum of support depends on clear dedicated budgets. The tools planners, advocates, policy makers and programmers need to be in place to double down on what works in HIV prevention, and, finally, end the epidemic.
New Podcast! Research Fundamentals: What is an endpoint?
We are delighted to share the next installment in our Px Pulse podcast series Research Fundamentals, explaining key concepts in HIV prevention research.
In this episode, Px Pulse host Jeanne Baron, and Matthew Rose, veteran HIV advocate and Director at Global Health Strategies, unpack what you need to know to understand endpoints in research.
Our series, Research Fundamentals is an addition to the regular schedule of programs on Px Pulse covering advances and challenges in HIV prevention research. Research Fundamentals offers short, concise and accessible conversations explaining scientific concepts in research that are key to understanding how HIV prevention science is advancing.
Endpoints are a crucial component in every clinical trial, but they are not always well understood. In addition, advocates can and should play a role, reviewing endpoints and interrogating how well the trial will serve communities that need HIV prevention.
Joining us to explore all this are:
- Dave Glidden, Professor of Epidemiology & Bio-statistics at UC San Francisco
- Erica Lessem, Senior Strategist for the New York City Department of Health and Mental Hygiene, former Deputy Executive Director, Treatment Action Group
- Meagan O’Brien, Senior Medical Director of Early Clinical Development & Clinical Experimental Sciences at Regeneron
Listen to the full podcast (9 minutes) to learn how endpoints are used in clinical research, why they change over time, and what matters most about endpoints for advocates and researchers alike. And here’s a transcript of the recording.
Endpoints are one element in evolving trial design for HIV prevention. Go to our dedicated page on trial design for more information and resources to engage with this fast-changing area.
Webinar on Omicron and HIV with Salim Abdool Karim
On December 15, the COVID Advocates Advisory Board (CAAB) and AVAC held a webinar about the Omicron variant featuring Salim Abdool Karim, director of the Centre for the AIDS Program of Research in South Africa (CAPRISA). Karim shared the latest updates and what questions are being pursued about Omicron, vaccines and any potential connections to HIV.
For background, read a commentary Karim co-authored with Quarraisha Abdool Karim in The Lancet, Omicron SARS-CoV-2 variant: a new chapter in the COVID-19 pandemic.
New Report: HIV Prevention R&D funding drops again
Today, we and partners are proud to release the annual HIV Prevention Research and Development Investments Report, with important findings for our collective advocacy. The report reveals a growing mismatch between the current promise of HIV prevention R&D, and continuing declines in the funding available. This decline affects both funding for research on new interventions and funding to expand access to existing prevention tools. The new report is based on outreach to 215 funders of HIV prevention R&D in the public, philanthropic and commercial sectors and includes 2020 funding data.
The latest data shows funding for HIV prevention R&D dropped by US$54 million (4.4 percent) in 2020. This second consecutive annual decrease is part of an eight-year trend of flat or declining funding for HIV prevention R&D.
The report also finds that financial support for HIV prevention R&D is almost entirely dependent on public sector funders, notably from the United States, and on one key United States-based philanthropic funder, the Bill & Melinda Gates Foundation. Commercial sector funding, already extremely low, dropped again in this year’s survey.
“These concerning trends in funding come at a promising but very demanding moment in efforts to control the pandemic,” said Mitchell Warren, executive director of AVAC, which coordinates the Resource Working Group with the International AIDS Vaccine Initiative (IAVI) and the Joint United Nations Programme on HIV/AIDS (UNAIDS). “Funding is declining just as the field confronts a new generation of opportunities and challenges.”
This high stakes environment includes: new products readying for introduction, such as injectable cabotegravir for PrEP and the Dapivirine Vaginal Ring; international support for ambitious new global targets for ending the epidemic; initial proof of concept of antibody-based prevention; and urgently needed new thinking for HIV vaccine development as recent trials have experienced setbacks and new technologies such as mRNA succeed against COVID-19.
Key findings from the report include:
HIV prevention R&D is highly overdependent on a few key funders, and much of the world is not contributing at the levels seen in prior years:
- HIV prevention R&D funding relies almost exclusively on the public sector, particularly the US public sector. The trend toward an overdependence on a small number of large investors, which the Working Group has surfaced and cautioned against in the past, intensified further in 2020.
- Globally, the public sector accounts for 86 percent of prevention R&D funding, with 92 percent of that coming from the US public sector.
- European public sector investments represent only 7 percent of the global total. While European public sector investment increased by 57 percent in 2020, it is still barely half of the US$124 million the European public sector contributed in 2009.
- The entire rest of the world accounted for only US$14 million, or just 1 percent of total public sector funding.
- Philanthropic funding, consisting almost exclusively of funding from the Bill & Melinda Gates Foundation, declined 20 percent in 2020 to US$127 million or 12 percent of the total global investment.
- Reported commercial sector support for HIV prevention R&D, already the lowest segment of investment, fell by 55 percent to US$31 million, or just 3 percent of the total, in 2020. While total commercial investment may be underreported, trends over time from the data collected show commercial sector investments is, by far, the smallest piece of the funding pie for HIV prevention R&D.
Funding dropped in 2020 across a number of key segments, including:
Preventive vaccine R&D: With two large-scale HIV vaccine trials underway, and dozens of new approaches under investigation, funding for preventive HIV vaccine R&D decreased by 5.5 percent or US$46 million in 2020 to US$802 million. While different European countries have increased or decreased their investments, overall European public sector investment in HIV vaccine R&D decreased 31 percent in 2020, to US$48 million.
R&D for PrEP, including pills, implants, injections: While uptake of oral PrEP grew substantially in 2020, and multiple recent research studies have demonstrated the potential impact of a range of PrEP options including long-acting injections, pills and implants, global investment in PrEP R&D declined 2 percent in 2020 to US$107 million. While US public sector donors increased funding for PrEP R&D by 5 percent, and commercial sector investment increased by 21 percent to US$24 million, neither was enough to overcome a 42 percent decline in funding from the philanthropic sector.
Voluntary Medical Male Circumcision (VMMC): As a number of studies affirmed the efficacy of VMMC over a decade ago, funding in the field is focused on implementation science, behavioral studies and advocacy and policy, each of which is vital to extending the reach and impact of this highly effective prevention tool. Yet investment in VMMC decreased by 37 percent to just US$6 million in 2020, almost all of which came from a single donor, the Bill & Melinda Gates Foundation.
Preventing vertical transmission: Prevention of mother-to-child transmission of HIV (PMTCT) remains a key prevention priority, but funding for PMTCT R&D decreased by 29 percent in 2020, from US$35 million to US$25 million. The decline is attributed to the loss of the Bill & Melinda Gates Foundation from the list of PMTCT R&D funders, and to decreases in funding from public donors. US public sector funding for PMTCT R&D fell 22 percent to US$22 million in 2020. European funding also fell more than 60 percent, from US$3.4 million in 2019 to US$1.3 million in 2020.
Only two areas of prevention R&D funding showed small increases in funding, including:
Treatment as Prevention (TasP): Long neglected in HIV prevention investment, funding for treatment as prevention (TasP) R&D increased from $1.7million to US$9 million in 2020. The increase came from philanthropy, notably the Bill & Melinda Gates Foundation (US$5 million) and the Wellcome Trust (US$1 million).
While TasP R&D funding is small overall, this increase is a hopeful sign that TasP may once again receive its appropriate focus as priority for HIV prevention research.
Microbicides: After multiple years of decline, investment in microbicide R&D registered a very small increase (0.4 percent or US$0.6 million) to US$145 million in 2020. Concerningly, there is even less diversity in microbicide funding than in HIV prevention R&D overall, with the public sector providing 99 percent of microbicide R&D resources.
While this tiny increase is a hopeful sign, it does not match the scope of the promise of microbicides. One key product, the Dapivirine Vaginal Ring, is now recommended by the WHO as an additional HIV prevention option. In addition, a range of promising microbicide strategies are under investigation. One, a 90-day dual-purpose vaginal ring designed to confer both contraceptive and HIV protection, was found to be effective in early testing.
This is the 16th annual report from the Resource Tracking for HIV Prevention Research & Development Working Group. Go to HIVResourceTracking.org to explore the key findings, funding trends, and previous reports in depth and follow the conversation on Twitter #HIVResearchFunding.
Speeding up Investments in Research and Development to Meet Public Health Needs
By Alice Kayongo, public health and human rights advocate, and senior policy advisor for WACI Health.
For over a decade, advocates for HIV prevention research have called for stronger political will and global solidarity towards a preventive vaccine and other prevention tools for HIV. COVID-19 has demonstrated how political will can help accelerate scientific breakthroughs. The COVID-19 vaccine is a case in point: science, political will and global solidarity came together to find a tool that has helped protect millions, and could protect billions if inequitable access issues are addressed.
As advocates call for action, it’s clear that greater domestic investment in health research and development (R&D) will be critical for improving health, equity and development. However, despite a disproportionately high burden of disease, Africa still lags in health R&D to address the region’s health challenges. We attribute this in large part to inadequate funding among other factors.
A recent publication, ‘Situation analysis report on the mobilization of resources for health research and development’, commissioned by Africa Free of New HIV Infections (AfNHi), WACI Health and Coalition to Accelerate & Support Prevention Research (CASPR), examines the financing problem of health R&D in Africa. It reports on progress but finds efforts have fallen gravely short of the target, with serious implications. This report comes at an opportune time, as COVID-19 puts a spotlight on the importance of health R&D and the need for greater domestic leadership and international commitment.
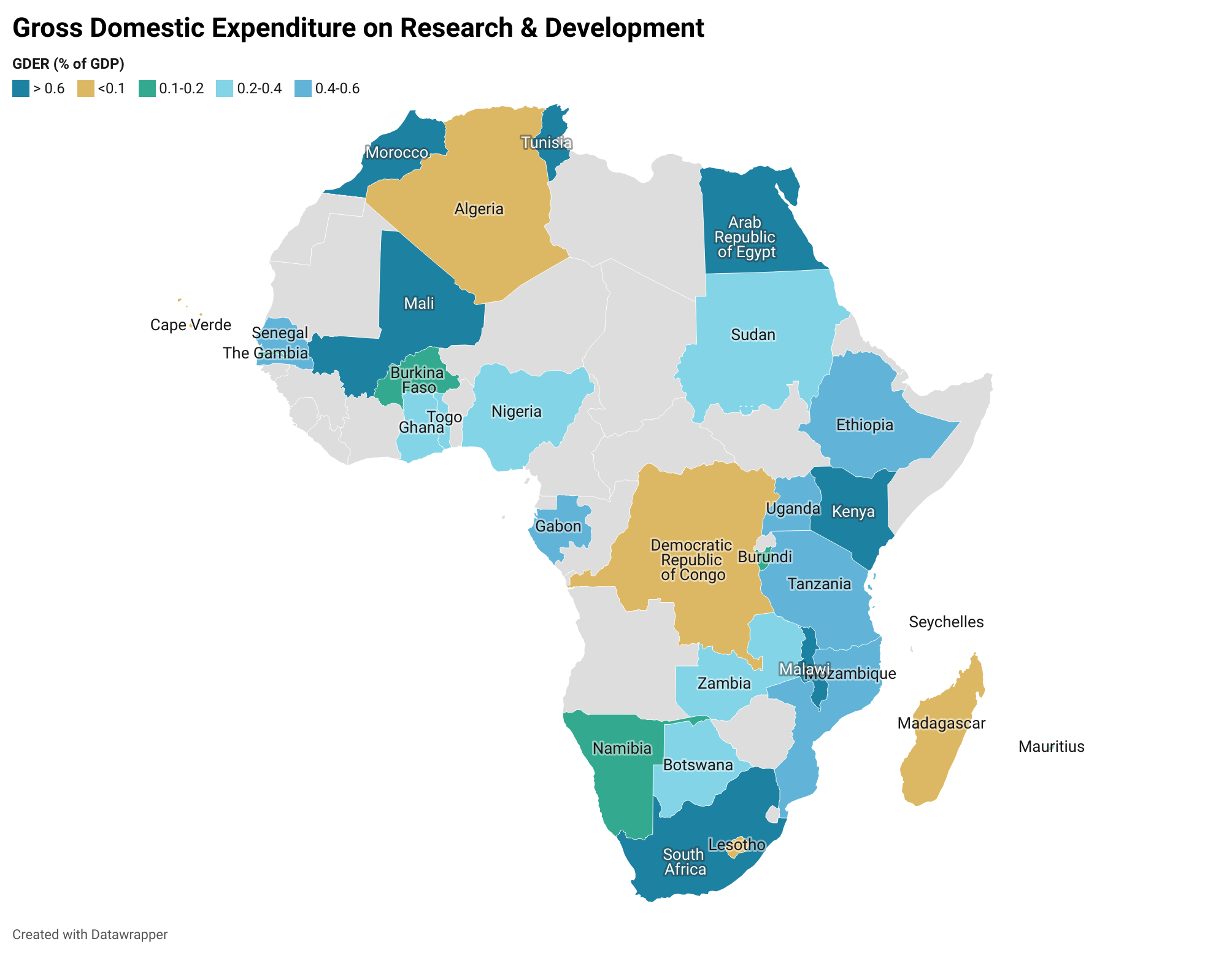
Accounting for over 15 percent of the world’s population, the continent bears 25 percent of the burden of disease at the global level, produces only 2 percent of the world’s research and only accounts for 1.3 percent of publications on global health. As explored in the report, regional commitments have been made to increase government spending on health R&D. For instance, the 2008 Bamako declaration calls on African governments to allocate at least 2 percent of budgets of ministries of health to research. Similarly, the 2008 Algiers Declaration calls on African governments to invest at least 2 percent of their national health expenditures and at least 5 percent of their external aid in projects and programs that build capacity and advance health research. However, despite such commitments, such investment remains gravely low in Africa.
Source: Simpkins (2019); Analysis by AfNHi Consultant
Based on the map above, domestic funding is inadequate for sustained and impactful research in health as most governments are investing less than 2 percent of their GDP, yet several health problems demand more investigation, arising from specific conditions, such as HIV, Malaria, challenges to maternal health, that are so widespread that they undermine public health at large.
Financing for health, especially R&D, relies heavily on foreign aid. This presents opportunities and challenges. Foreign aid brings skills, technology transfer, infrastructure for research and other resources. But the challenges are important to consider. Foreign aid can put funder research priorities ahead of host country priorities. Foreign aid can also be tied to a lack of local ownership, exploitative research partnerships, focus on publications vs. investing in the capacity for sustained research and development, and undermining the independence and success of innovative domestic research institutions. In some countries where governments have committed significant investment for health R&D, it’s often not financial, and often not useful. For financial investments, for instance, in 2017, the SciDev.Net reported that Uganda committed 30 billion Uganda Shillings (about USD 9Million) to support innovation and technology, and the first round of grants would be given mostly to individuals who had products in place. But critical voices point out that giving these funds to individuals rather than institutions undermine efforts to develop a sustainable ecosystem for innovation, one that supports individuals and a system that nurtures them.
Some of the sharpest criticism among African scientists, ministers, advocates and other observers say research in Africa too often can be experienced by African scientists as extractive, a ‘slave model’, where foreign funders reap the benefit of African intellectual labour but leave behind few benefits for ordinary Africans. Researchers are sustained by their own governments to continue their work with salaries and operational costs. Those same researchers advance proposals to foreign donors, often in consortia, and may individually benefit with publications, promotion, peer recognition and presentations at international conferences. But the true impact of their labour—the field advances with new tools, products and interventions—is not felt at home. An African scientist quoted in Scidev.net noted “Maybe we should have an incentive system structured differently in research institutions. An innovation is a public good with commercial value and industrial application, but a publication has knowledge value. When we reach an extent where they will use commercial output from innovators other than publication, we shall see more innovations come out.”
The Situation analysis report on the mobilization of resources for health research and development finds that Africa has made progress towards financing for health R&D, especially in the past decade, but many African countries still have significant gaps to address. Of all the sampled countries, the report showed that Malawi had the highest proportion allocated to research in general at 1.06 percent of GDP, while Kenya had 0.79 percent, Rwanda 0.66 percent and Eswatini 0.27 percent. The report has clear recommendations – for policy makers and civil society organizations to address the challenges.
The reports findings and recommendations call for:
a. Increased government funding for health and research, which signals government leadership and commitment, and encourages greater investment from domestic partners
b. Strengthening existing laws, regulations and policies and enacting new ones where needed to guide research
c. Increase the influence of research on government policy by locating research closer to political power and aligning research priorities between researchers and governments
d. Governments must lead and facilitate collaboration between government and the private sector to fund and conduct contextually relevant health research
e. Research in Africa must prioritize benefits to Africans in research and development Incorporate robust accountability structures for efficient use of research resources
f. Advocating collectively for an enabling democratic environment for effective research
g. Strengthen cross-sectoral partnerships among CSOs to advocate for health research and development
h. Strengthen the advocacy capacity of CSOs in health financing and health research
The following are key strategies that advocates should consider:
1. Demonstrate to governments how specific investments are cost-effective, bring health and socioeconomic benefits, and enable broader governmental objectives
2. Explains the consequences of not investing in health R&D, including a slowing economy, reluctance of business and funder entities to invest, a “brain drain” of science and medical professionals, and cascading losses of the R&D benefits to other countries
3. Choose a collaborative approach to ensure that key government officials and policymakers view advocates as assets, partners and problem solvers with whom relationships can be formed towards the realization of health objectives and the mobilization of resources
4. Partner with experts in disciplines such as economics, public finance, business and international development for the strongest possible advocacy for health R&D
5. Strengthen capacity to interrogate and track government funding and actual expenditure on health R&D
The report concludes that governments, among others, in low and middle income countries (LMICs) must prioritize and ensure budgetary allocations to health R&D, beyond the non-financial investments. Budget commitments have the potential to attract additional investments by partners and demonstrate political will. It’s time to champion this work! Join us for the Biomedical HIV Prevention Forum (BHFP) Pre-Conference at ICASA, scheduled for December 6, 2021 at 12:25pm SAST as we dive deep into this conversation and draw collective action points.
A Look at HIV Prevention at ICASA
The biennial International Conference on AIDS and Sexually Transmitted Infections in Africa, ICASA, will take place this year December 6 – 11, in Durban South Africa and virtually (register here). ICASA launched over two decades ago to offer an African platform for leaders, scientists, activists and community to frame the challenges, track the science and push innovation in the HIV response. As COVID-19 continues to ravage communities, disrupt the response to HIV and force the world to confront profound inequity in global health, partners across the field have been dogged in their advocacy to lose no ground to COVID-19, adapt and demand solutions that work.
“The world has missed crucial targets for HIV prevention, and COVID-19 has further undermined hard-won progress. But the unparalleled innovation of these last 20 months have offered new models of collaboration, adaptation and investment. We are coming together now to exchange what we’ve learned and embolden each other. Because the time to act is now, when the entire world has been forced to see the impact of inequity, and that no one is safe until everyone is safe.” Rosemary Mburu, WACI Health executive director, CASPR partner and featured speaker at the Biomedical HIV Prevention Forum and other sessions at ICASA.
ICASA 2021 will feature a host of events, and AVAC and partners will be there exploring in depth where and how the response to the dual pandemics must dismantle the structural barriers to health faced by key populations, intensify demands for robust domestic and global funding for health, accelerate and expand access to prevention options, integrate HIV services with sexual and reproductive health, and more. Scroll down for a roadmap to ICASA’s prevention sessions, and details on sessions and events that we don’t want to miss.
- Go to our dedicated page for ICASA 2021 for updates on this schedule
- Download the ICASA Prevention Roadmap
- Follow the conversation on Twitter
Sessions of Interest
Sunday, December 5
Youth Pre-conference
09:00-16:00 SAST / 11:00-15:00 EAT / 02:00-09:00 ET
Open to all young people, join this event for workshops, break-out sessions and discussions on youth leadership, resilience and innovation toward the 2030 targets for ending the epidemic. Organized by Y+. Free and open to all.
Register here.
Gay, Bi-sexual and Men who have Sex with Men (GBMSM) preconference
11:00-16:30 SAST / 12:00-17:30 EAT / 04:00-09:30 ET
Join health and human rights advocates at this combination virtual and in-person event to take stock of the status of advocacy and activism, kickstart a conversation on sustainability for GBMSM programming across Africa, and define aspirations for health and rights going forward. Organized by Black Gay Men Connect, PAI, UKPC, Fierté Afrique Francophone, APHA, GALZ, GHPN Ke and AVAC. Free and open to all.
Register here.
Monday, December 6
PSI: Realizing the Benefits of U=U in National HIV Programs
10:40-11:25 SAST / 11:40-12:25 EAT / 03:40-04:25 ET
This session explores the prevention impact of supporting people living with HIV to remain virally suppressed, and the importance of programming that understands that undetectable HIV is untransmissible, U=U.
Examining How National Laws/Policies Impact the Global AIDS Response
11:35-12:15 SAST / 12:35-13:15 EAT / 04:35-05:15 ET
This special session will draw from insights and analysis from the HIV Policy Lab and feature a panel discussion on the intersection of policy, equity and ending the epidemic.
The Biomedical HIV Prevention Forum pre-conference
12:25-15:00 SAST / 13:25-16:00 EAT / 05:25-08:00 ET
This event, available for virtual participation, will bring together front-line providers, advocates, community, researchers and policy makers to frame what’s needed now for HIV prevention in Africa, in the context of COVID-19. Discussions will include updates on scientific advances, and how to strengthen African-led advocacy for prevention research. The gathering will also put a spotlight on advocacy to beef up domestic spending on health systems and research in Africa.
Register here.
PROMISE Collaboration: Launching PrEP-it 2.0
13:30-14:05 SAST / 14:30-15:05 EAT / 06:30-07:05 ET
This satellite symposia explores an innovative online platform for planning, monitoring and evaluating PrEP agents. This session will showcase how the platform has been used to set evidence-based targets, project costs and estimate impact.
Tuesday, December 7
No Prevention, No End. Taking the lead in implement the 2025 HIV Roadmap
10:42-11:27 SAST / 11:42-12:27 EAT / 03:42-04:27 ET
This session looks at the recommendations from the Global HIV Prevention Coalitions Working Group to reach key goals by 2025. Discussion will also focus on the role of political and community leadership and successful practices in the HIV response.
Resource Mobilization for HIV, TB, Malaria and Pandemic Preparedness
13:30-15:30 SAST / 14:30-16:30 EAT / 06:30-08:30 ET
For those attending ICASA in person, this event looks at factors shaping health financing, and extends the ongoing conversation on increasing domestic spending on the health sector. At the Southern Sun Elangeni & Maharani, sponsored by the GFAN Africa and the Global Fund.
Wednesday, December 8
Providing Alternative HIV Prevention Tools For High-risk Populations in Eastern Africa. Dapivirine Ring Could be a Game Changer
10:30-11:15 SAST / 11:30-12:15 EAT / 03:30-04:15 ET
A look at the potential impact of this new addition to the HIV prevention toolbox and next steps to make it accessible.
The Dual Prevention Pill (DPP): What advocates need and want to know
17:00-18:00 SAST / 18:00-19:00 EAT / 10:00-11:00 ET
Join AVAC and AYARHEP for a discussion on the Dual Prevention Pill (DPP), a combination pill in development that prevents HIV and pregnancy. If approved, it would be the first multipurpose prevention technology (MPT) available since male and female condoms. What information on the DPP do you need to inform your advocacy? We hope you’ll join the discussion!
Thursday, December 9
Biomedical HIV prevention for men in a time of a pandemic: Amplifying our gains and increasing in scale, impact, and sustainability of Voluntary Medical Male Circumcision (VMMC) beyond 2021
13:33-14:18 SAST / 14:33-15:18 EAT / 06:33-07:18 ET
This satellite session looks at the impact of VMMC and how to sustain it. A panel discussion will focus on action steps needed from implementers, policy makers, civil society and other decision-makers.
Conducting 2nd Generation surveillance during a pandemic in Nigeria: lessons learnt
13:33-14:18 SAST / 14:33-15:18 EAT / 06:33-07:18 ET
Nigeria’s efforts to maintain HIV services during COVID-19 offer key lessons for the field.
What’s new from WHO: “Guidelines for validation of elimination of mother to child/vertical transmission of HIV, STI and Hepatitis
14:30-15:15 SAST / 15:30-16:15 EAT / 07:00-08:15 ET
Updates on the WHO guidelines and their implications for HIV prevention and HIV services.
The highlights above represent an extraordinary body of work that is urgently needed right now. And this is just a small sample. To follow happenings and discussions from ICASA watch this space, and follow #ICASA2021 on Twitter.