A string of announcements today coming from AIDS 2022 mark a potential turning point for HIV prevention and for the global health field at large, but a grave question hangs in the balance: which way will we go?
The big news yesterday was a dire warning: the launch of the UNAIDS report, In Danger, documenting what HIV advocates have known: that during the earliest phases of the COVID-19 pandemic, progress against HIV did not just stall, it actively lost ground.
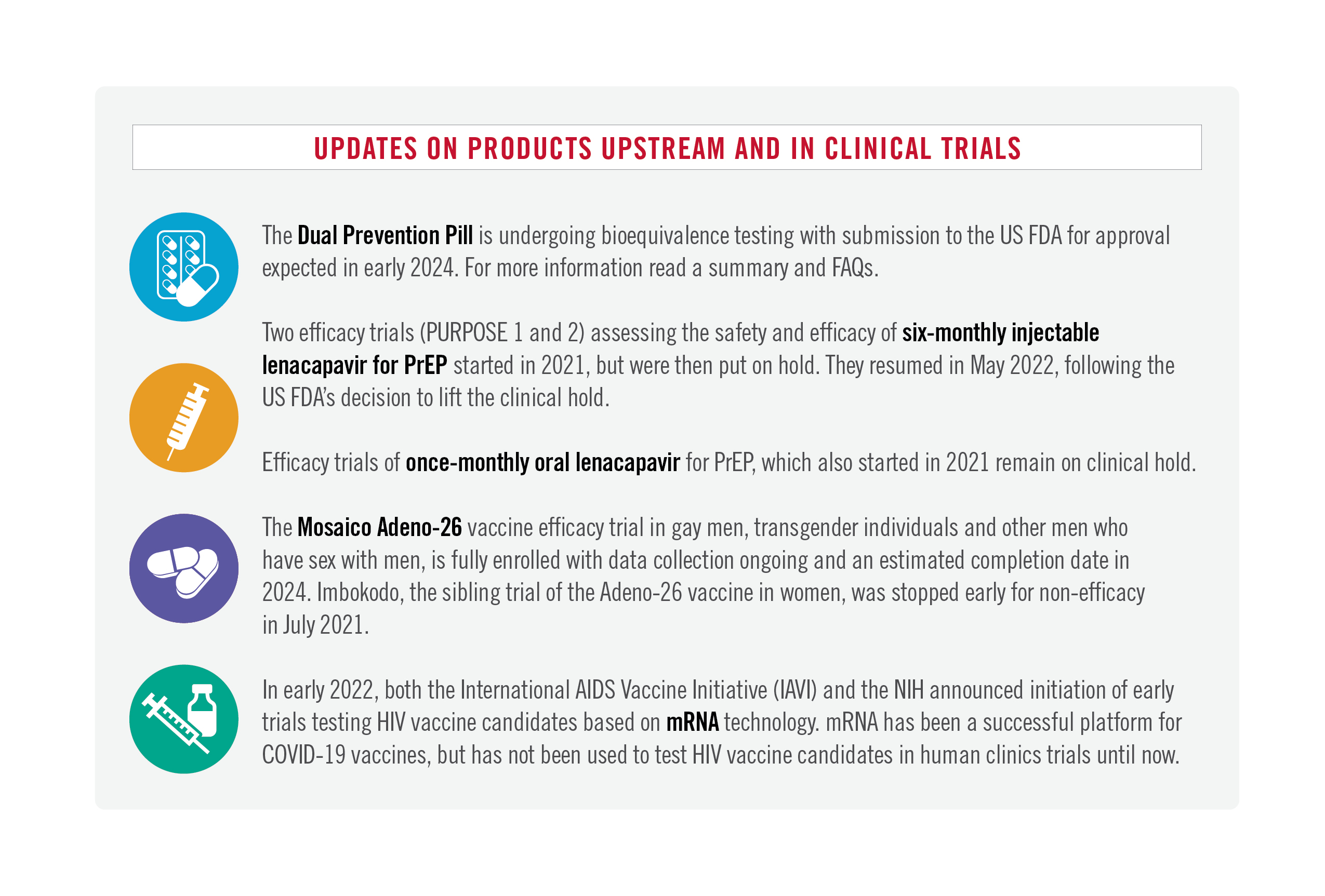
The news today included: promising developments with new data from the HPTN 083 and 084 trials testing injectable cabotegravir for PrEP, which re-confirmed its safety and efficacy; WHO announced new guidelines adding injectable PrEP as an additional prevention option; the drug-maker for the first approved product of injectable PrEP (cabotegravir as PrEP), ViiV, announced an agreement with the Medicines Patent Pool to provide a voluntary license and begin the process of identifying generic manufacturers; and a new coalition was announced, convened by Unitaid, WHO, UNAIDS and the Global Fund, with AVAC serving as the secretariat, to collaborate with civil society, normative agencies, governments, and funders, in accelerating access to affordable longer-acting PrEP.
In the whiplash of announcements and headlines, it’s vital to understand how much is at stake at this moment.
Will proven biomedical options become feasible choices for people who need and want them? Will policies, programs and investments come together for equitable access? It’s up to all of us.
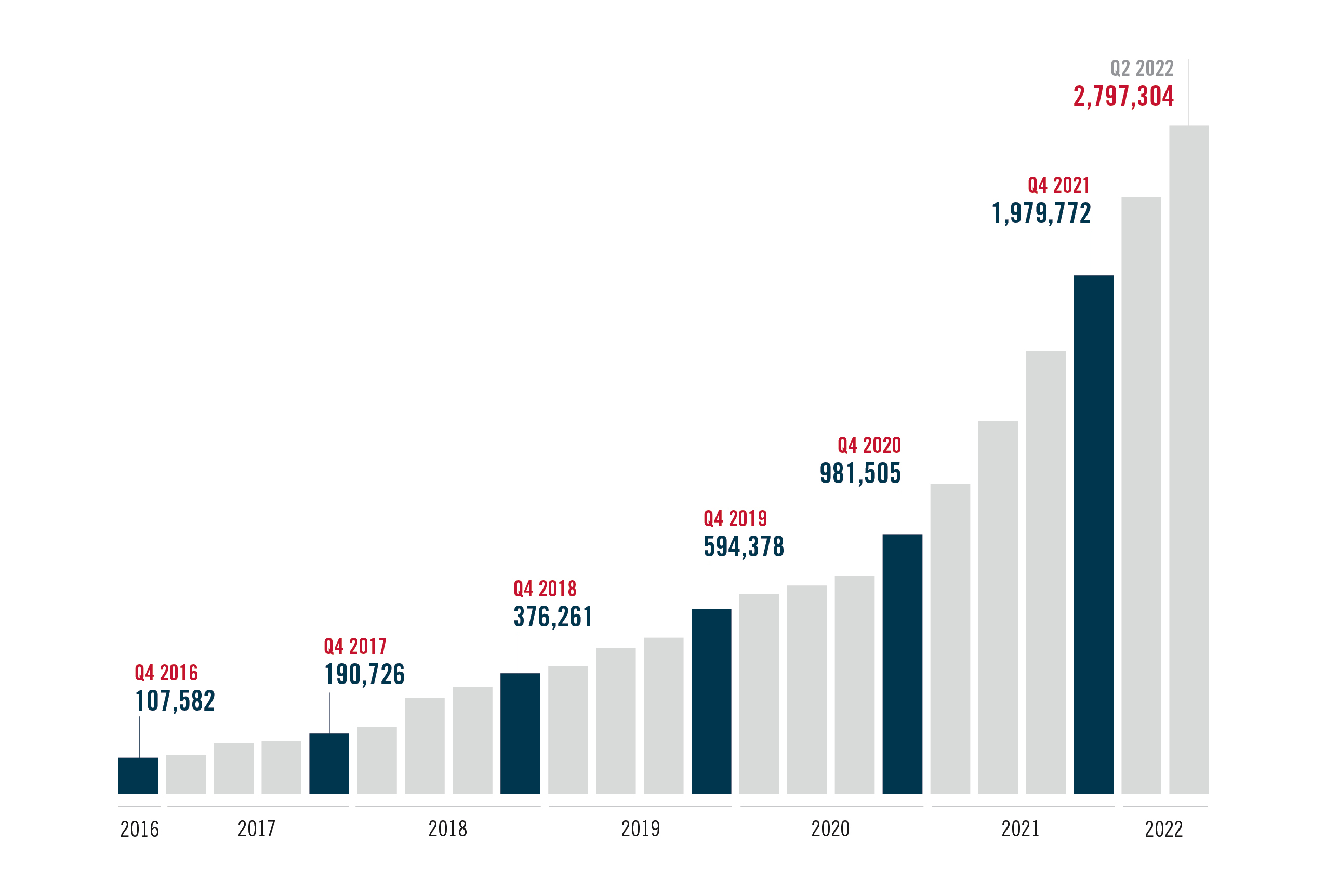
PrEP has an important role to play in reaching targets and accelerating an end to the epidemic. But new AVAC data show only around 2.7 million people have initiated use of oral PrEP since it was introduced ten years ago, falling short of the 2020 target of three million users – and representing only a fraction of the estimated number of people who need it and could benefit from it. Now, the dapivirine vaginal ring and injectable cabotegravir (CAB for PrEP) offer additional options – and an opportunity to re-imagine HIV prevention.
The news from Montreal show promise and potential to develop and deliver new PrEP options faster, smarter and with greater equity than ever before.
AVAC’s Plan for Accelerating Access and Introduction of Injectable CAB for PrEP provides a comprehensive view of all the moving parts involved in delivering this new PrEP option and identifies priorities for ensuring time is not wasted and opportunity is not squandered. The plan focuses on learning the lessons from the first ten years of delivering oral PrEP. It shows how to move faster, more strategically, and with greater coordination— to maximize the impact of injectable CAB for PrEP today, and to overcome access challenges for new PrEP options in the future.

There is enormous work ahead, and it will require all proven prevention methods be available in a marketplace of choice. And these efforts must integrate the involvement of all stakeholders, including civil society, to hold each other accountable. We encourage you to read the plan, or our summary of it, and follow events in real time on Twitter at #AIDS2022. And learn more about the dapivirine vaginal ring and injectable cabotegravir (CAB for PrEP).