December 19, 2024
This year, the HIV prevention community witnessed a landmark moment with the results of the lenacapavir (LEN) for PrEP PURPOSE 1 and 2 trials, which showed nearly complete protection against HIV. Named as “breakthrough of the year” by Science Magazine, LEN’s every-six-month injectable dosing has the potential to transform the HIV response, but only if it is rolled out with speed, scale and equity. Gilead Sciences, the developer of LEN, just began the process to submit applications to national regulatory agencies to market LEN for PrEP. WHO is also beginning to develop guidelines for LEN for PrEP. And just this week, PEPFAR and the Global Fund announced a coordinated effort to reach two million people with access to LEN. So, the clock is ticking and achieving this vision—and significantly reducing global HIV infections requires coordination at every level.
We released a new framework, the Gears of LEN for PrEP Rollout, which provides more details about all the moving parts needed to accelerate equitable access – and the necessary lubrication to ensure the world does not squander this opportunity. Read a new op-ed penned by Jirair Ratevosian and Mitchell Warren in Devex describing this process and read more below about this framework and tools and strategies to support the advocacy needed.
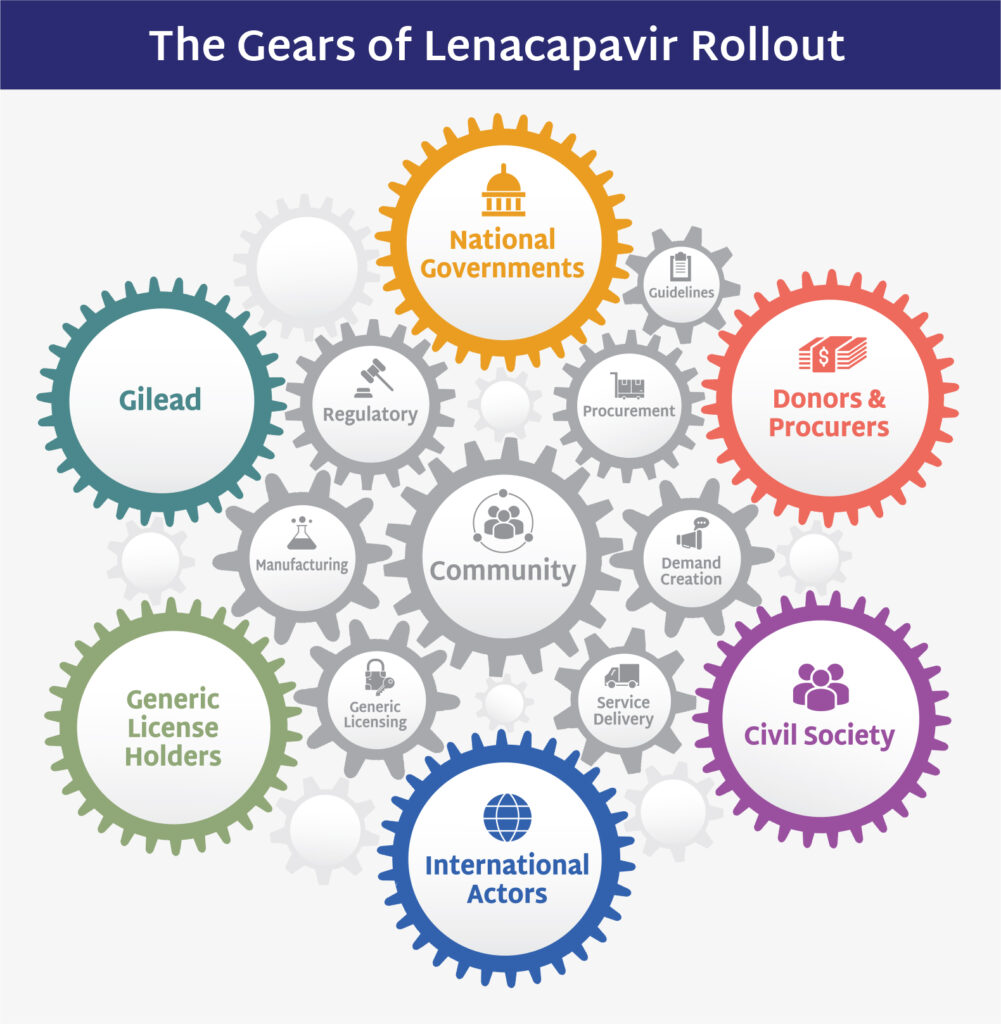
The Gears of Lenacapavir for PrEP Rollout underscores that each stakeholder—whether governments, donors, civil society, or manufacturers—must function like the gears in a finely-tuned clock. Everyone has a role to play–each component is essential, and only by working together in synchronization can we keep to time and ensure a seamless and impactful rollout.
Recent LEN for PrEP News
- The world has a new HIV prevention drug. Let’s use it, Devex
- Gilead Submits New Drug Application to U.S. Food and Drug Administration for Twice-Yearly Lenacapavir for HIV Prevention
- Global Fund, PEPFAR Announce Coordinated Effort to Reach 2 Million People with Lenacapavir for PrEP to Significantly Reduce Global HIV Infections
- Global Fund, PEPFAR to reach 2 million people with the 6-monthly long-acting injectable Lenacapavir for HIV prevention, Global Prevention Coalition
- 2024 Breakthrough of the Year, Science Magazine
- Gilead Agrees to Allow Generic Version of Groundbreaking HIV Shot in Poor Countries, New York Times
Resources
- The Lens on LEN: The basics on injectable lenacapavir as PrEP, Advocates’ Guide
This advocates’ primer provides background on the product, the trials (including results of both PURPOSE 1 and 2), key questions, and next steps.
- Lenacapavir: The case for investing in delivering HIV prevention, Podcast
AVAC’s PxPulse podcast goes deep on LEN for PrEP. Recorded just days before Gilead’s announcement that PURPOSE 2 also found very high efficacy, Dr. Flavia Kiweewa, a principal investigator of PURPOSE 1, lays out the research findings and what they mean. And Chilufya Kasanda Hampongo of Zambia’s Treatment Advocacy and Literacy Campaign and Mitchell Warren of AVAC talk about how to change a long history of squandered opportunities to get rollout right.
- Update on Lenacapavir for PrEP, Webinar recording
AVAC hosted a webinar focused on updates for the PURPOSE trials. Gilead provided an overview of the PURPOSE 1 and 2 trial results and insight into the status of PURPOSE 3, 4, and 5.
Choice, Not Miracles
AVAC Communications Director, Kenyon Farrow breaks down the potential of LEN to make choice a reality. View the video.
Instagram Reel
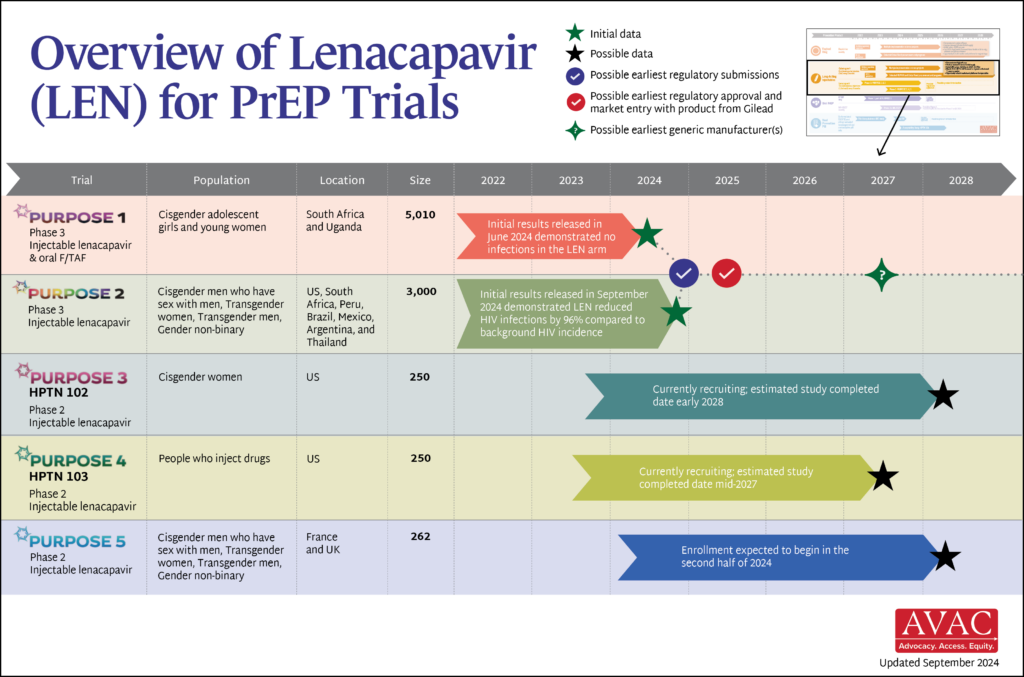
An Overview of Lenacapavir PrEP Trials
This graphic shows the latest status of all five trials including the groundbreaking results of PURPOSE 1 and PURPOSE 2. Download the graphic here.
Additional Infographics
- Moving a product to the real world
- LEN generics—Can we go faster?
- PrEP price comparison
- PrEP initiations by country worldwide
Lenacapavir isn’t just another medication; it could be a catalyst for transformation. Together, we can seize this pivotal moment to revolutionize HIV prevention, paving the way for a future where innovative tools like lenacapavir reach everyone who needs them.