United States financing decisions, global governance and scientific innovation collide as US lawmakers advance the fiscal year 2026 (FY26) bills. The foreign assistance budget, passed by the House on Wednesday, if enacted, would restore some global health funds in a broader context of uncertainty, as US bilateral “America First” health MoUs advance without transparent plans for implementation or accountability. At the same time, abrupt reversals by the US Department of Health and Human Services (HHS) of troubling health policy decisions underscore the instability of the Administration’s decision-making process. These developments are taking shape amid momentum around lenacapavir for PrEP, which was approved by the Brazilian regulatory agency and received additional funding from Unitaid to reach more populations in South Africa and Zambia.
US Reverses Harmful Health Policy Decisions After Outcry
The US rescinded one, and possibly two, very troubling health policy decisions following outcry from communities and experts. Health and Human Services (HHS) reversed plans that would have canceled billions of dollars in funding for overdose prevention and substance use treatment programs. In addition, The Guardian reports that an HHS-sponsored hepatitis B vaccine trial in Guinea-Bissau has been halted following serious ethical concerns related to informed consent, standards of care, and potential exploitation in a low-income setting. While Scientific American reports that the study may still be ongoing, in both cases, sustained objections from public health experts, advocates, and affected communities played a decisive role in forcing scrutiny of deeply troubling decisions.
IMPLICATIONS: These rapid announcements followed by abrupt reversals and confusion expose deeply flawed leadership at HHS, where ideological hostility to evidence-based public health has driven attempts to dismantle lifesaving programs and advance vaccine-skeptical agendas. At the same time, they offer a clear reminder that advocacy works. Public outcry from communities directly affected by overdose deaths, global health experts, ethicists, and civil society played a critical role in helping to prevent harmful actions. While these outcomes prevent immediate harm, they do not erase the broader instability facing US health policy.
READ:
- Controversial US study on hepatitis B vaccines in Africa is cancelled—The Guardian
- CDC Will Continue a Controversial Vaccine Study in Africa—Scientific American
- H.H.S. Reverses Decision to Cut $2 Billion for Mental Health and Addiction Services—New York Times
- 24 hours of chaos as mental health grants are slashed then restored—NPR
FY26 US Global Health Funding Update
The US House and Senate Appropriations Committees released their negotiated FY26 funding bill covering National Security, the State Department, and Related Programs (previously referred to as the State, Foreign Operations, and Related Programs/SFOPs bill). Also released was a summary statement of what’s in the bill, including timelines, reporting and other requirements for specific budget allocations. Together, these signal a return to a bipartisan appropriations process and, if enacted, provide basis to push back against unilateral cuts by the Administration. The House has approved the bill, and it now heads to the Senate. Overall, approximately $50 billion is allocated for foreign assistance, $20 billion more than the President’s Budget Request, with $9.4 billion directed toward global health programs. Importantly, Congress maintained funding for core global health priorities, including HIV, tuberculosis, malaria, polio, family planning and reproductive health, neglected tropical diseases, Gavi, and UN agencies such as UNAIDS, UNICEF, and UNFPA. As KFF’s analysis shows, the bill reflects strong bipartisan congressional support for global health even as the Administration sought deeper cuts and eliminations across several programs.
IMPLICATIONS: The FY26 bill underscores ongoing uncertainty in US global health priorities and financing. As AVAC’s Mitchell Warren told Devex, “Almost a year since the US administration decimated the U.S. Agency for International Development … Congress is coming back with a bill that rejects the administration’s shortsighted decisions last year. But this is just a start and major challenges remain: Will both houses of Congress approve the bill? Will the President sign it? And, perhaps more importantly, will the President actually spend Congressional appropriations and accept at last that it is Congress, and not him, who has the power of the purse?” Congress’s decision to protect key investments signals continued bipartisan recognition that global health programs, including HIV prevention and treatment, are essential to US and global security. However, the overall funding reduction leaves programs in limbo, prolonging instability for implementing partners and countries that rely on US support.
READ:
- Unexpected global health wins in the US foreign aid bill—Devex
- US lawmakers strike $50B foreign assistance deal, surpassing Trump’s plan—Devex
- Friends welcomes Global Fund support in fiscal year 2026 appropriations legislation—Friends of the Global Fight
- Congress to reject Trump global health cuts—Politico
- US Congress backs Gavi, the Vaccine Alliance, despite Trump admin cuts—Devex
- Global Health Funding in the FY 2026 National Security, Department of State and Related Programs (NSRP) Conference Bill & Explanatory Statement—KFF
America First Global Health MoUs Under Scrutiny
Discussion and scrutiny is intensifying around the five-year bilateral Memorandums of Understanding (MoUs) signed with the US under the America First Global Health Strategy. Fifteen nations have now signed these agreements and more than $16 billion has been committed. The Implementation Planning Period, where operational details would be worked out, including who would deliver services, where, and for which populations, was supposed to happen upon signing of the agreements through March 31, 2026. However, as many tracking these agreements suggest, completing the MoUs does not guarantee a detailed, transparent, or people-centered implementation strategy.
IMPLICATIONS: Without clear implementation plans that specify roles, funding flows, safeguards, and mechanisms for civil society engagement, billions in health assistance could be deployed in ways that weaken coordination, sideline communities, and fail to reach those most affected. As the Implementation Planning Period unfolds, sustained oversight will be essential to ensure these agreements translate into measurable health gains.
READ:
- KFF Tracker: America First MOU Bilateral Global Health Agreements—KFF
- The America First Global Health Strategy and Pooled Procurement—KFF
- US Foreign Aid Planning Tool Drafts Put America First, Health Impact Last—To End A Plague Again Substack
- Senior Science Advisor at the Bureau of Global Health Security and Diplomacy Addresses MoU Process—To End A Plague Again Substack
- Citizens will pay the price of health data as a bargaining chip in Africa—Devex
- Copper for HIV drugs: Inside Trump’s new aid trade—The Telegraph
Unprecedented Momentum for Lenacapavir for PrEP
Unitaid, a convener of the Coalition to Accelerate Access to Long-Acting PrEP, announced a new $31 million commitment to accelerate access to injectable lenacapavir for PrEP (LEN) in South Africa and Zambia. The investment aims to expand delivery of LEN beyond traditional clinic settings and reach populations often left behind, including young women and key populations. In addition, Brazil became the 14th regulatory agency to approve LEN this week, and Gilead submitted an application to Peruvian regulatory authorities, marking nine pending regulatory approvals (see AVAC’s regulatory tracker).
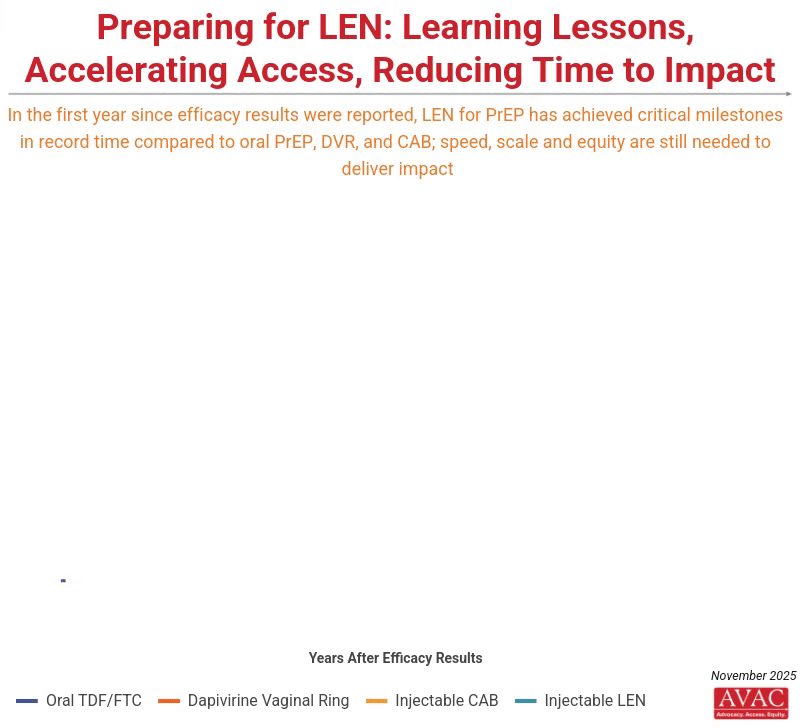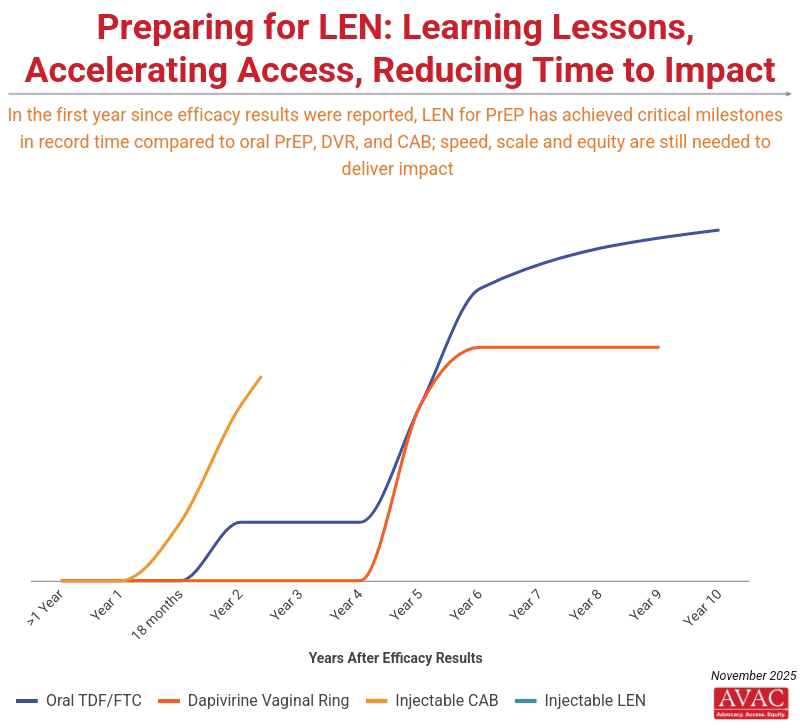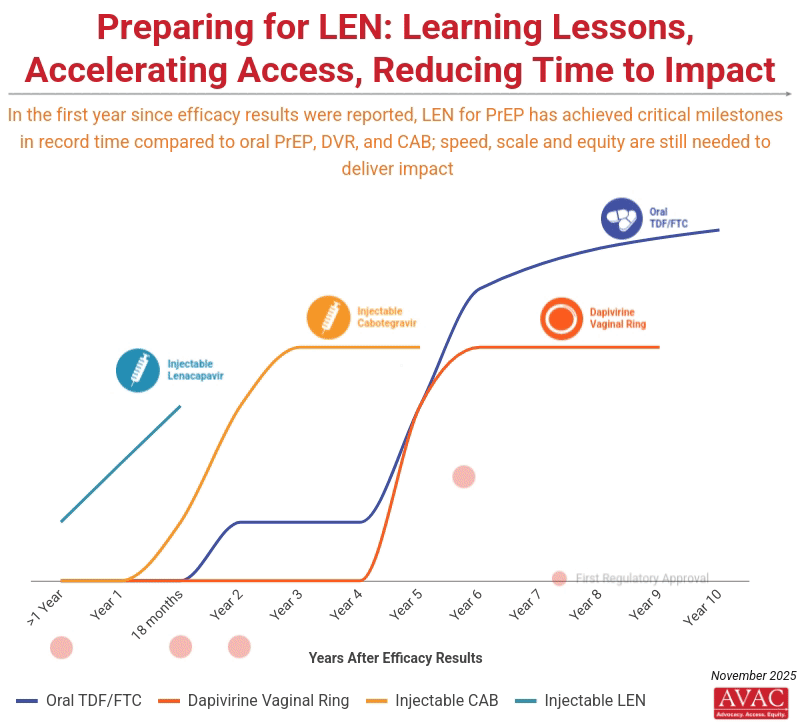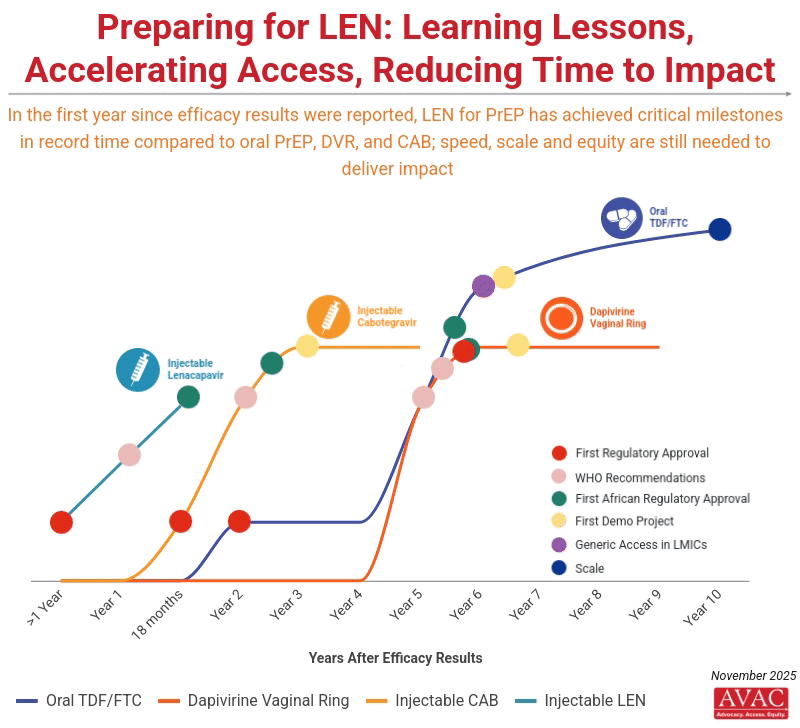
IMPLICATIONS: The momentum of LEN activity to date is unprecedented. In less than 18 months, the field has moved from initial LEN efficacy results to a series of critical milestones (including this week’s announcements) that took over seven years with oral PrEP and three years with injectable CAB. This momentum underscores both what is possible—and what is at risk. Rolling out LEN with speed, scale, and equity must be an uncompromising priority if this breakthrough is to change the trajectory of the epidemic. Without robust community engagement, intentional planning, sustainable financing, and strong delivery systems, high costs and weak infrastructure could confine LEN to a limited number of countries and populations. While this has not yet translated into infections averted at scale, it provides clear proof that faster pathways are possible, and that this accelerated model must now become the new benchmark for how effective global health technologies (not just HIV prevention options) are designed, developed and delivered.
READ:
- Unitaid will provide funds for South Africa and Zambia to widen access to Gilead’s HIV prevention drug—STAT
- Anvisa approves new drug with biannual injection to prevent HIV—Latin America News
What We’re Reading
- US cuts to HIV programs in sub-Saharan Africa pose global risk, experts say—CIDRAP
- Inside Trump’s $11 billion health plan to replace “neo-colonial” USAID—Axios
- When Programs Vanish: The Unseen Consequences—Clinical Infectious Diseases
- Medical Groups Will Try to Block Childhood Vaccine Recommendations—New York Times
- Hospitals and doctors are ignoring RFK Jr.’s new vaccine schedule and relying on pediatricians’ guidance instead—STAT
- After a year of chaos, US CDC’s global health work hangs in the balance—Devex
- Member States to Discuss US Withdrawal from WHO as Failure to Pay Fees Violates Agreement—Health Policy Watch
- Investor behind Moderna says U.S. policy is ‘taking a sledgehammer to our miracle machine’—STAT
- mRNA technology for the prevention and treatment of HIV-1 infection—Nature
- Development leaders must win the narrative battle or disappear—Devex
- NIH loses yet another leader as heart, lung and blood director exits—Fierce Biotech
- Judge orders HHS to restore funding for children’s health programs as lawsuit continues—STAT
- Most Vaccine Hesitancy Can Be Successfully Overcome, New Lancet Study Finds—Health Policy Watch
- Pazdur warns that politics, ‘chaos’ are damaging FDA—STAT
- Gates Foundation unveils $9 billion budget and plans to cut staff—Associated Press