Welcome to the Daily Snapshot for Day 2 of the HIV R4P prevention conference. As with our Day 1 coverage, this is a selective, whirlwind tour through some of the day’s sessions—all of which are available via webcast 24 hours after they took place. There’s lots more information and insight in the conference’s liveblog and the Twitter feed #HIVR4P.
Morning Plenaries
“Epidemiology has been called the basic science of prevention,” said Johns Hopkins researcher and President of the International AIDS Society, Chris Beyrer, at the outset of a comprehensive, eloquent look at the prevention needs for “key populations”, which he defined as populations with disproportionate burden of disease and lack of access to services. Beyrer, who dedicated his talk to health providers caring for Ebola patients, is a passionate advocate of both human rights and biomedical prevention, and his talk is a valuable contribution to the discourse that merges these sometimes siloed arenas.
Beyrer also said that he “could not wait for the PrEP era to begin.” In fact, the era took a leap forward while the plenaries were taking place, as the French research agency ANRS released a press release announcing that its IPERGAY trial, which had been designed to evaluate intermittent PrEP use, had found evidence of efficacy and was going to end its randomized, placebo-controlled design and offer oral PrEP to all participants.
Australian advocate, ethicist and researcher Bridget Haire was up next, with a talk that spanned a decade of biomedical prevention research—from the 2004 controversies regarding PrEP trials in Cambodian sex workers—through to the present day, when issues about post-trial access to strategies that show benefit is emerging as a key and sometimes thorny topic.
Pontiano Kaleebu, leader of the Medical Research Council in Uganda, was the final speaker, providing a really concrete picture of ongoing work in Uganda designed to provide targeted, effective combination prevention—including immediate initiation of ART in sex workers and members of fishing communities.
Other Presentations
Maturation, parallel paths, strange behavior—HIV and antibodies
B-cell immunity is a major focus of this conference, as noted in yesterday’s blog. Today’s morning antibody presentations continued the exploration of factors in HIV-positive individuals that affect the development of broadly-neutralizing antibodies (BNAbs)— antibodies that block the activity of multiple strains of HIV. In a Monday session [SY01.04], Garnett Kelsoe (Duke University) remarked on how “strange” these HIV-specific BNAbs are relative to other antibodies. One aspect of this strangeness is that the B-cells that produce broadly neutralizing antibodies against HIV have gone through many more rounds of somatic hypermutation than is typically seen in other infections. Somatic hypermutation is the process by which the B cell’s antibody-producing genetic code is progressively revised, potentially leading to an increase in the affinity of the antibody for its target. The steps of this revision are also called a hypermutation “pathway” that leads to mature, BNab-produce B cells. There isn’t one maturation pathway—there are many. And the conference is shedding light on what’s known about what influences these pathways for different BNAbs. Yesterday, Kelsoe gave a fascinating talk on the role of immune “tolerance” in shaping B-cell driven responses to HIV. Tolerance refers to one of the ways the immune system distinguishes self- (safe, not needing an immune response) from non-self (potential threat) substances in the body. Kelsoe explained that BNAb-producing B cells may be eliminated at tolerance “checkpoints”–and that some of their unusual characteristics may reflect steps taken to overcome these checkpoints. Today, Jinal Bhiman (National Institute for Communicable Diseases, South Africa) [OA12.01] and Elise Landais (IAVI) [OA12.02] described how viral evolution in people with HIV affects the development of BNAbs in these same individuals. An afternoon session [SY06.02] included more exploration of the topic—including a look at “systems serology” which is getting better at predicting antibody efficacy.
RV144 Trial and Follow-on Activities Get a Boost
Several of today’s presentations gave a sense of the what’s happened since the 2009 announcement that the Thai vaccine trial known as RV144 provided modest efficacy in Thai volunteers. Rapid analysis of available samples identified immune factors associated with reduced risk among vaccine recipients including a non-neutralizing antibody. Since the end of the trial, some of the Thai vaccine recipients have been “reboosted”—meaning they received additional immunizations of one or both components of the regimen. Volunteers who received the regimen six years ago were reboosted in hopes of prompting antibodies to develop into BNAbs. (Antibody development and maturation is dependent on a highly-specialized interaction between the virus and the immune system–what parts of the virus are “seen” by the virus affect what antibodies evolve). Michael Anthony Moody (Duke University) presented data showing that this strategy did prompt a development of BNAbs providing insights into how the regimen might be improved (OA12.06 LB). Siriwat Akapirat (AFRIMS) reported on measurable immune responses in rectal secretions of re-boosted volunteers—mucosal immune responses weren’t measured in the original study [OA11.05LB]. Finally, Glenda Gray (Medical Research Council, South Africa) presented on the immunogenicity of the RV144 regimen in South African volunteers in the HVTN 097 trial [OA11.06LB]. Vaccines can have different effects depending on gender, body mass index and background immune activation—so vaccine-induced immune responses can and do vary by geography. The good news, as Gray described, is that the South African volunteers had responses that were as good—if not better—than RV144 volunteers, paving the way for the planned efficacy trials slated to start in 2016.
Truth, lies and teapots
Anyone interested in adherence, trust-building between trial participants and trial staff, and the ways that participants decide what to disclose, and when, about product use should grab a cup of tea and watch the full session [OA15]. Tea is relevant because it’s the basis of an innovative, simple graphic used to explain the concept of pharmacokinetics (PK) (detection of blood drug levels) to VOICE trial participants in VOICE-D—a protocol that engaged participants after the trial found no evidence of benefit in any of the arms—to understand why that finding might have happened. Participants reported on their product use and then discussed it again, after receiving PK data. The findings are important and intriguing.
Hormones and HIV
Do hormonal contraceptives, including Depo Provera, affect women’s risk of HIV? And if so, why? The data to date are mixed—some studies say yes, others say no. Today’s session [OA20] added more information to this uncertainty. A South African study suggested a five-fold increase in HIV risk associated with Depo use, perhaps because progestogen increases the number of HIV target cells in the cervix. (So does the natural form of the hormone, progesterone.) But this study wasn’t designed to answer the question directly, and other high-quality studies haven’t found the effect. What’s needed, said conference co-chair Helen Rees, in remarks from the floor, is the planned randomized trial known as ECHO, which would evaluate HIV acquisition in women using three different methods. Rees, who is one of the principal investigators on ECHO, said the trial “appeared” to be close to receiving full funding—a hopeful sign of progress towards certainty.
Long-acting injectables and other new strategies
In a session [SY07] that provided overviews on a range of next-generation strategies (rings, gels, injectable ARVs), Ian McGowan (MTN) provided some initial data from pharmacokinetic and pharmacodynamics studies of long-acting injectables, including findings of different drug levels in vaginal and rectal tissue and secretions.
Double-edged swords, competing priorities and a roadmap for communication
Today’s session on the Good Participatory Practice Guidelines for biomedical prevention research [OA19] featured compelling talks on how media engagement with LGBTs can help—or hinder—an effective human rights response; on a Kenyan model for joint priority-setting between LGBT groups and researchers; implementing GPP in the FACTS 001 microbicide trial; and more.
Penis enthusiasts and other ways to reframe the reality of HIV prevention today
A tweet-friendly session provided both soundbites and deep discussion of what prevention needs to be truly effective. Advocate and leader of the International Rectal Microbicide Advocates, Jim Pickett, said that it was essential to change the discourse. Let’s stop stigma around sex and pleasure, he said. Instead of the term “whores”–which has been used to label gay men and other MSM in the US who choose to use PrEP–he suggested “penis enthusiasts.”
There’s much more that hasn’t been described in this update—full sessions on prevention of mother-to-child transmission, mucosal immunity and more. We welcome your thoughts and questions—from at the conference or those following from afar. Be in touch!
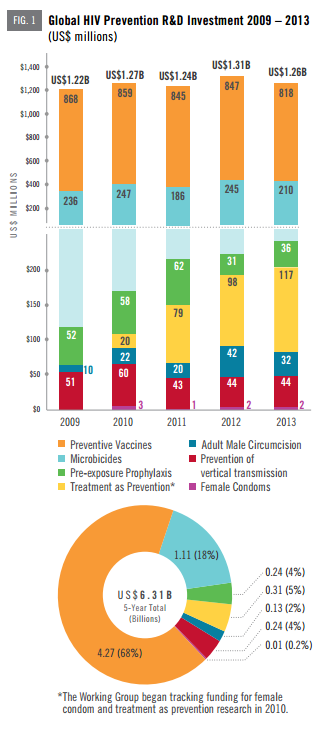 Progress toward new tools to prevent HIV infection—including vaccines, microbicides, the use of antiretroviral treatment as HIV prevention, pre-exposure prophylaxis (PrEP) and a host of other options—is being presented and discussed at the inaugural
Progress toward new tools to prevent HIV infection—including vaccines, microbicides, the use of antiretroviral treatment as HIV prevention, pre-exposure prophylaxis (PrEP) and a host of other options—is being presented and discussed at the inaugural