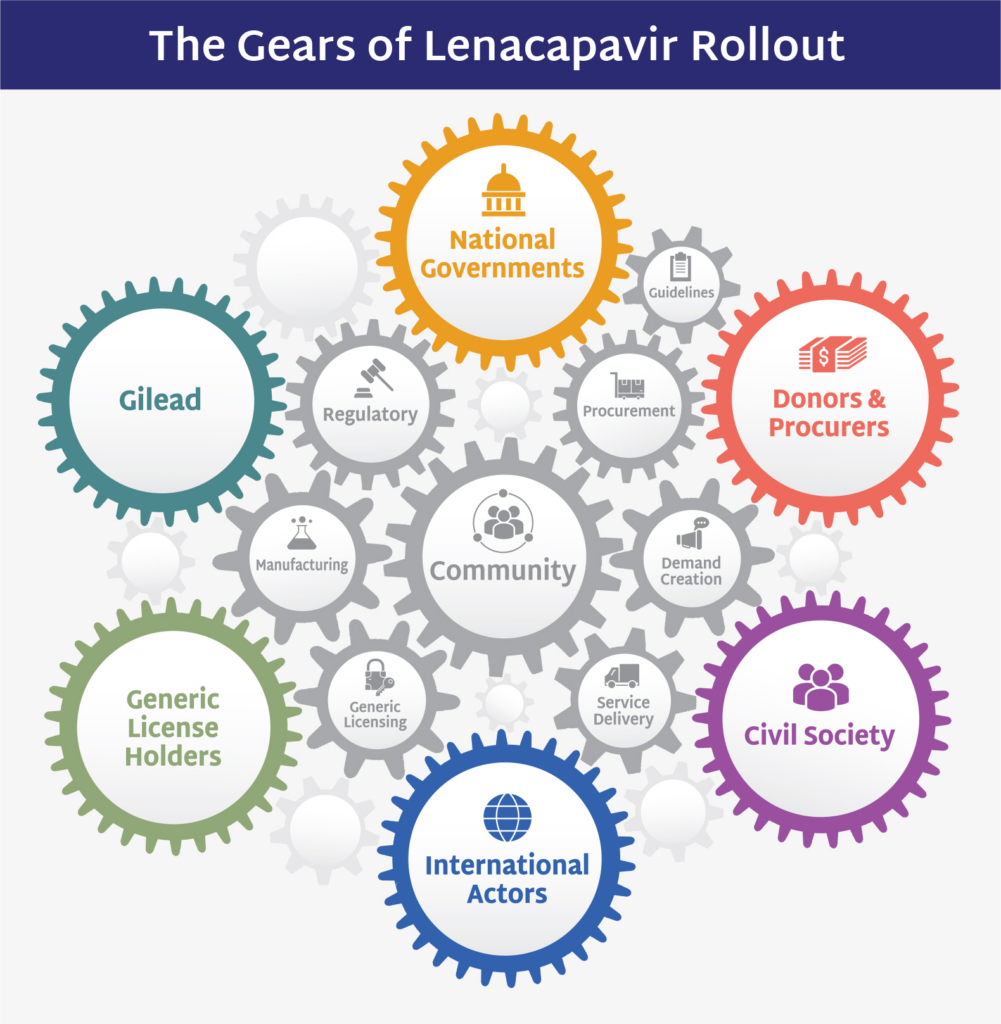
The Good Participatory Practice (GPP) Guidelines have been shaping and improving HIV prevention research since 2007. They provide a global reference guide for ethical and effective stakeholder engagement, helping ensure the priorities of trial participants and their communities are centered in clinical trials and broader research agendas.
One year ago, AVAC published the Good Participatory Practice (GPP) Body of Evidence, an online clearinghouse of tools, best practices and analyses showcasing the power of GPP. Here, we bring you a report from the year since – how this clearinghouse of resources continues to demonstrate the value of GPP, and concrete examples from 2024 of GPP’s impact on major research agendas and mechanisms. Read on for highlights.
- Critical Learnings from GPP: The Body of Evidence Webinar Series
- GPP in Action: Recent Examples of Influencing Research Programs
- Elevating GPP: WHO’s New Clinical Trials Guidance

Critical Learnings from GPP: The Body of Evidence Webinar Series

Throughout 2024, AVAC and partners facilitated a series of webinars in collaboration with The Global Health Network, Wellcome Trust, and WHOfeaturing resources housed in the Body of Evidence. These conversations expanded the traditional understanding of GPP – highlighting that GPP is not just about trial implementation; that its practices evolve from product discovery to delivery and are important at every step of the way; and that monitoring and evaluation are complex and critical nuances are required to ensure its meaningful application. Look out for a final webinar on elevation of GPP in global clinical trials guidance in 2025. The full recordings and presentations are on the AVAC website.
A Few of our Favorite Moments from the Webinar
Our great leader Nelson Mandela said, ‘everything that is done for me, without me, is done against me,’ and we really must see our community members as having a role beyond that of as just a potential trial participant but to engage them right from the beginning, from the protocol design, from all our planning pre-study, the conduct of the study, and most important to the dissemination of the results – whether they be positive or negative. — Dr. Michelle Temeris, University of Capetown
How can we craft research questions so that when we have an answer at the end of the day it’s really something meaningful and impactful to communities? We can answer scientific questions that might be interesting to a researcher but at the end of the day that doesn’t get us very far if it’s not also equally impactful for community. — Sarah Read, US National Institute of Allergy and Infectious Diseases
Thinking about community engagement moving forward we need to think about building relationships over time and beyond particular studies. We need to make sure that we’re not only giving accurate information but we’re also listening and responding to issues that are being raised in the course of our interactions. — Sassy Molyneux, KEMRI-Wellcome Trust, University of Oxford
One really important perspective would be to monitor the impact that engagement has on the trial, the way it’s run. That would be a really important aspect of monitoring and evaluation – to make note of the real changes that community stakeholders can have on the way trials are selected in the first place but also modified to make them appropriate. — Alun Davies, Global Health Network
GPP in Action: Influencing Research Programs
Advocates’ Consultation on Merck’s Monthly Pill Program
In recent years HIV prevention efficacy trial design has become one of the hottest topics. As the HIV prevention toolbox improves, researchers, statisticians, and regulators grapple with the best way to incorporate these options into efficacy trials. The key to all of this, they say? Community.
 Enter GPP! In June, AVAC convened a community and advocates’ consultation with Merck around their program testing MK-8527 as a monthly pill for PrEP. With an efficacy program on the horizon, Merck set out to consult with communities – before any other stakeholder – about issues like choice of a comparator arm, the evolving standard of prevention, and how a trial could best reflect the reality of implementation in peoples’ countries, communities, and own lives. Consultation members consolidated feedback that is now being fed into Merck’s protocol development. Priorities included a design that would get to an efficacy answer most efficiently, but that would incorporate contextual issues of prevention choice as possible. Participants concluded that a monthly pill would be an important addition to the prevention toolkit, and thus support for the research program. But they also expressed ongoing frustration around community support for research that does not translate into access for their communities.
Enter GPP! In June, AVAC convened a community and advocates’ consultation with Merck around their program testing MK-8527 as a monthly pill for PrEP. With an efficacy program on the horizon, Merck set out to consult with communities – before any other stakeholder – about issues like choice of a comparator arm, the evolving standard of prevention, and how a trial could best reflect the reality of implementation in peoples’ countries, communities, and own lives. Consultation members consolidated feedback that is now being fed into Merck’s protocol development. Priorities included a design that would get to an efficacy answer most efficiently, but that would incorporate contextual issues of prevention choice as possible. Participants concluded that a monthly pill would be an important addition to the prevention toolkit, and thus support for the research program. But they also expressed ongoing frustration around community support for research that does not translate into access for their communities.
Watch this space for further updates on the MK-8527 program, as engagement continues through protocol development, trial planning, implementation, and beyond!
Pediatric Adolescent Virus Elimination (PAVE) Community Advisory Board (CAB)
Communities have been a key stakeholder advancing HIV cure research from the bench to early phase clinical trials. GPP has been the guiding principle as engagement has moved further upstream.
The Pediatric Adolescent Virus Elimination (PAVE) Community Advisory Board (CAB) is an example of this impact as the only group dedicated to advancing an HIV cure in pediatric populations. Through its digital Voices Project featuring young people with HIV, clinicians, caregivers and researchers, the CAB has raised awareness of the research among youth in Sub-Saharan Africa who engaged with ministries of health to push for the inclusion of children in research. They also worked with PAVE investigators to simplify and convey complex scientific and ethical issues inherent in cure research.
As research moves from the bench to the clinic, the CAB and community partners, will continue to play a strategic role in shaping future protocol designs and addressing community support needs.
Elevating GPP: WHO’s New Clinical Trials Guidance
In September, 2024, the World Health Organization published their new Guidance for Best Practices for Clinical Trials. The guidelines state that centering patient, participant, and community engagement will lead to more efficient and equitable trials. Input from AVAC and partners, including Wellcome Trust, The Global Health Network, University of Oxford, and others helped ensure the new guidance centered the importance of community engagement in creating more equitable and actionable clinical trials research.
Save the Date

On December 13, 2024, WHO is hosting a webinar, WHO launches new clinical trials guidance – What do I need to know? Registration is free, and during the webinar WHO will highlight key areas of change in the guidance for everyone involved in clinical trials.
Since AVAC and UNAIDS launched the GPP guidelines in 2007, the science and politics have grown ever more complex – and GPP implementers have continued to adapt, evolve and engage. We are committed to continuing our efforts to curate insights and resources, including further building out the Body of Evidence, and to support our collective advocacy for ethical and effective stakeholder engagement throughout clinical trials, research agendas and implementation in the months and years to come.