By Jeanne Baron
World AIDS Day in 2025 comes at a moment of unprecedented change. Over 60 years of investment in global health, forty years of innovation and progress in the fight to end the HIV epidemic, and many of the critical programs that provide access to HIV prevention in high burden countries are in disarray and at risk of collapse. As the US government withdraws and redirects resources, the just-launched UNAIDS report shows the number of people living with HIV is predicted to increase from 40 to 50 million people by 2050, unless HIV incidence reduces dramatically. Yet, in the midst of the chaos, there’s a historic opportunity to defeat HIV by scaling-up the breakthrough technology of long-acting injectable PrEP.
As governments and HIV prevention champions around the world scramble to reimagine a people-centered HIV response and build anew, what will it take to finally achieve targets to drive down incidence and reach epidemic control?
1. Fulfill the promise of science with accelerated, equitable access to injectable lenacapavir (LEN) for PrEP, delivered at scale
Science and advocacy have delivered astounding progress in developing biomedical options for HIV prevention. Injectable LEN for PrEP is moving from research to rollout faster than any product before.
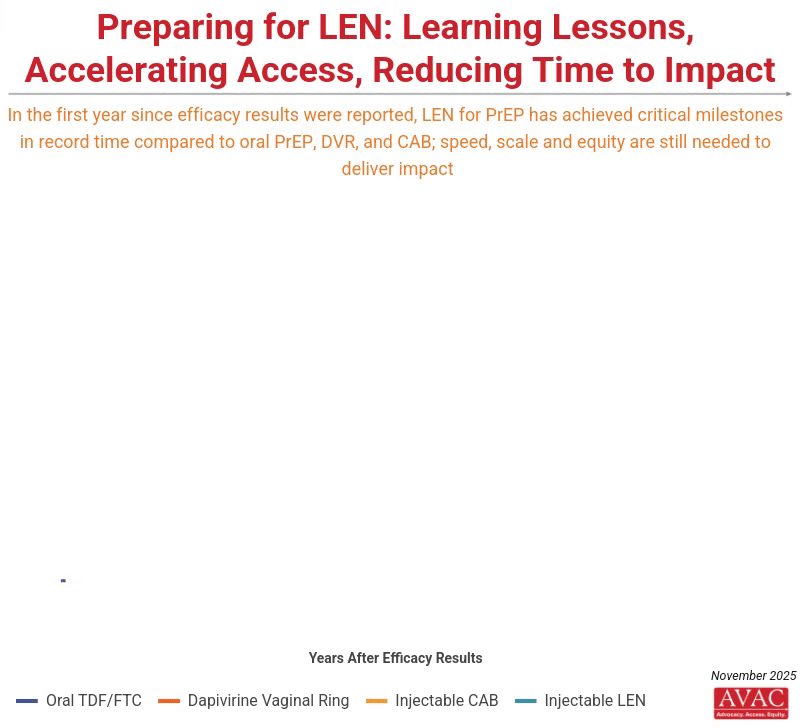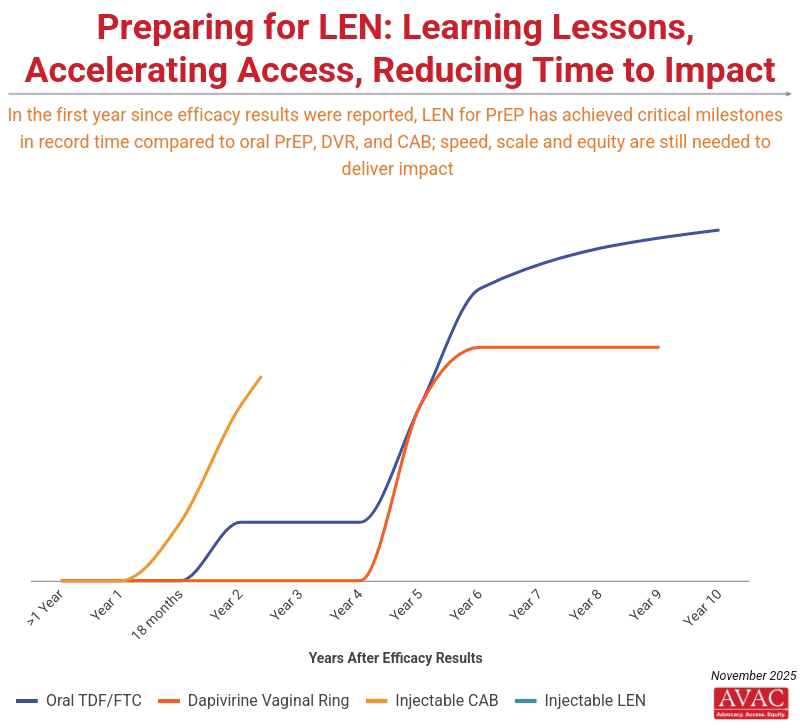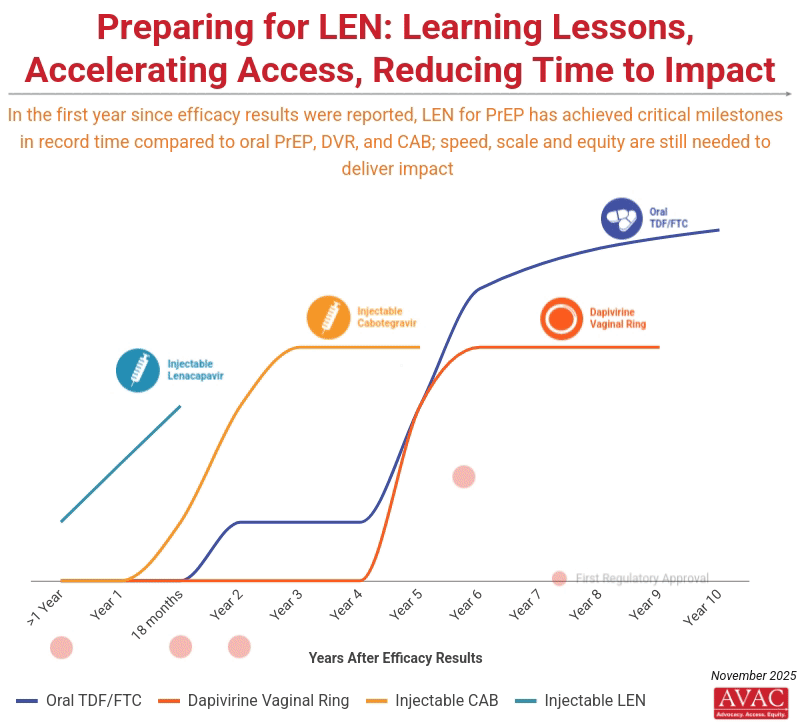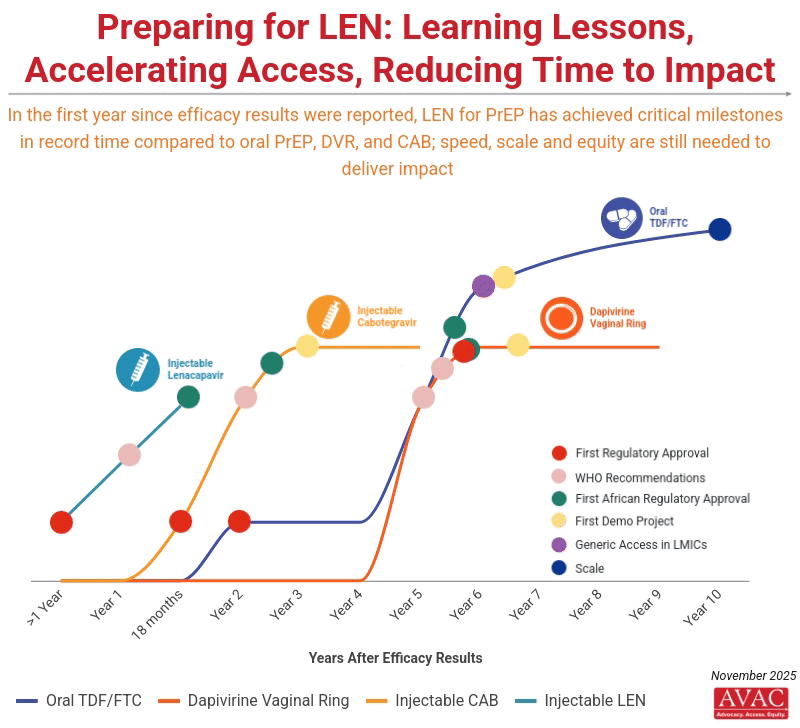
Current commitments from the Global Fund and PEPFAR to provide doses for two million people by 2028 in 12 initial countries represent a good start, but fall far short of reaching targets to achieve impact, which calls for 20 million PrEP users by 2030.
Making LEN for PrEP available for all who need and want it is possible. Coordination among all stakeholders, and a shared priority to achieve epidemic control, can build a sustainable market for LEN that drives volume up and prices (and HIV incidence) down; puts communities at the center of program planning and implementation; is supported by inclusive policies; and integrates with sexual and reproductive health services that offer choices in HIV prevention to meet diverse needs. To learn more about who and what must come next to realize this potential, check out AVAC’s suite of LEN resources and track updates on rollout with our long-acting PrEP dashboard.
2. Expand, strengthen and sustain community and civil society leadership in the HIV response
Engaged advocates are fundamental to the success of HIV prevention R&D and rollout and to the larger global health movement. Involving community, civil society and global health advocates too little or too late has taught the field valuable lessons. Community leadership was on full display at last week’s Global Fund replenishment. Informed and supported community leadership has reshaped and improved both clinical trials and the broader landscape of policies, planning and programs for HIV prevention. From the strategic organizing and monitoring by the Coalition to build Momentum, Power, Activism, Strategy & Solidarity (COMPASS) Africa, to the policy wins and mentoring successes of AVAC’s Fellows & Advocacy Navigator programs, to the high-impact reach of Coalition to Accelerate & Support Prevention Research (CASPR), these initiatives represent highly effective models for community engagement. Stay tuned for detailed reports on the impact of these models in the weeks ahead.
3. Double down on the research pipeline for HIV prevention and a People’s Research Agenda
The US government’s attack on HIV research has been just as evidence-free, ideological and devastating as the drive to dismantle foreign assistance. With epidemic control in sight against one of the fastest mutating viruses ever known, HIV prevention scientists and advocates know that now is the time to press on the gas, not stall out. From vaccines and bNAbs to multipurpose technologies (such as the Dual Prevention Pill), to novel long-acting PrEP (such as a monthly PrEP pill), advancing the development of options to meet diverse needs and offer choice is key to effective HIV prevention and ending the epidemic.
We have been marshalling the evidence and the organizing to defend HIV science and research. In September, months of teach-ins, tools development, tracking and collaboration culminated in the 24 Hours to Save AIDS Research marathon. Read more about the event—and what comes next—in our new blog, 24 Hour Marathon to Save HIV Research: A Global Call to Action. (Access resources for advocacy demanding sustained support for HIV R&D and view the videos here.)
As the new year approaches, there’s no doubt what needs to happen: deliver effective options with speed, scale and equity; invest robustly in developing the options we still need; and center the leadership of communities at every level of the HIV response. The world has every capacity to end the HIV epidemic in our lifetime, now we must summon the political courage to do it.




