There will be no issue next week. The NewsDigest will return on July 12, 2019. Happy Independence Day to those celebrating and a wonderful week to all!
The Weekly NewsDigest will return July 12
ECHO Study Results and Beyond: What’s Next
In the days since the leaders of the ECHO Study announced their findings, AVAC has produced a number of resources to inform advocacy and action.
Px Pulse
Tune in to a special episode of AVAC’s podcast, Px Pulse. The ECHO Trial Results: Time to Act features two veteran women’s advocates from Kenya and South Africa, Jaqueline Wambui and Yvette Raphael, who talk about what the high rates of HIV in the trial mean for advocacy now. Helen Rees and Nelly Mugo, members of the ECHO trial leadership team, explain the results and their implications, and James Kiarie of WHO shares the importance of the WHO guidelines and more. (And for background on the trial, you can also check out our pre-results podcast – The ECHO Trial: Preparing for Action.)
For the full podcast episodes, highlights and resources, visit avac.org/px-pulse. Subscribe on Apple Podcasts to catch every episode.
Understanding Results
AVAC has published a comprehensive guide to interpreting the results of the trial in Understanding the Results of the ECHO Study. You’ll find concise information on the trial’s background, design and results, and a full section on next steps such as the WHO process for updating its guidelines and what you can do to get involved.
Webinar: What do the ECHO Study results mean for African women?
In case you missed it, AVAC and ICW-EA hosted a webinar on June 27th with Jared Baeten and Tim Mastro from the ECHO Consortium, and James Kiarie from WHO. In a discussion moderated by ICW-EA’s Lillian Mworeko, speakers discussed implications of the findings and took questions from members of the ECHO Global Community Advisory Group and other women’s health advocates.
Use these tools and find more at www.avac.org/echotrial to join the movement demanding informed-choice and expanded, integrated options for HIV prevention and sexual and reproductive health.
June 27 Webinar: What do the ECHO study results mean for African women?
[UPDATE: Recording and slides from the webinar are now available below. And a link to Understanding the Results of the ECHO Study is now available as well.]
Please join women and our allies for a global webinar, Thursday, June 27, 9-10am EDT / 3-4pm SAST / 4-5pm EAT, on shaping the post-ECHO agenda for comprehensive sexuality and reproductive health and rights. This emerging agenda will inform an upcoming WHO meeting on this critical subject. Please join the leaders of the Civil Society Working Group on HC-HIV, members of the ECHO Global Community Advisory Group and young women advocates for this discussion with WHO and the ECHO Consortium Management Team members.
On June 13, the Evidence for Contraceptive Options and HIV Outcomes (ECHO) study released its results. The study was designed to evaluate the risk of acquiring HIV among HIV-negative women who used one of three contraceptive methods: depot medroxyprogesterone acetate-intramuscular (DMPA-IM), also known as Depo-Provera, a copper intrauterine device (Cu-IUD), and a levonorgestrel (LNG) implant, also known as Jadelle. The study found that there was no substantial difference in HIV risk among women using any of these three methods. These results mark an important step for women’s health—the findings provide evidence that WHO and national ministries of health will hopefully use as they develop policies and programs that directly impact women’s lives.
What do the ECHO study results mean for African women: A webinar organized by the Civil Society Working Group on HC-HIV (co-convened by AVAC and ICW-EA) — Thursday, June 27
Recordings & Slides: YouTube / Jared Baeten and Tim Mastro’s Slides / James Kiarie’s Slides.
Prepare for the discussion with resources available on www.avac.org/echotrial, which are also highlighted below:
- HIV incidence among women using intramuscular depot medroxyprogesterone acetate, a copper intrauterine device, or a levonorgestrel implant for contraception: a randomised, multicentre, open-label trial— The Lancet
- How Should We Listen to ECHO? — The Lancet commentary
- Watch the presentation of the ECHO study results from the SA AIDS Conference
- FP2020 and AVAC held a webinar, A Roadmap for Results: Understanding the ECHO Study Results, with the ECHO team following the results announcement. The webinar explained the trial, provided topline results, outlined next steps, and offered key advocacy messages to help all stakeholders understand the findings. Watch here.
- Visit the ECHO page for a statement from the Civil Society Working Group on HC-HIV on the study results, as well as a range of other public statements on the results and their implications.
In the coming days, AVAC will release Understanding the Results of the ECHO Study, a document designed to help advocates understand some of the issues related to the ECHO trial, the questions it was designed to answer, its findings and next steps. AVAC will also release the next episode of our Px Pulse podcast, where you will hear from members of the ECHO Trial team and leading women’s advocates discuss what they think about the results, so be on the lookout!
Paving the Road for Rollout
Jeanne Baron is AVAC’s web editor and producer of Px Pulse.
This diverse set of some of the latest resources on PrEPWatch.org will help program implementers from a variety of contexts plan for the rollout and scale-up of PrEP. Added to the already extensive tools and resources available on PrEPWatch.org are: country-specific Situation Analysis for Kenya, South Africa and Zimbabwe; Health Care Providers’ Knowledge, Attitudes and Practices (KAP) Relevant to Oral PrEP Service Provision to AGYW in Zimbabwe; PrEP Costing Guidelines; The Common Agenda for the Dapivirine Ring, and The Dapivirine Ring Introduction Matrix.
Latest additions:
- Country-specific Situation Analysis for Kenya, South Africa and Zimbabwe, have been developed to assess factors that are crucial to the expansion of PrEP rollout. Each report explores the strengths, challenges and progress to date on planning and budgeting, supply-chain management, the status of infrastructure and human resources places where people can obtain PrEP, and how people learn about PrEP and are supported in taking it.
- Health Care Providers’ Knowledge, Attitudes and Practices (KAP) Relevant to Oral PrEP Service Provision to AGYW in Zimbabwe: This KAP study among health care providers in Zimbabwe explored questions about PrEP services for adolescent girls and young women. Health care providers serve as gatekeepers, encouraging or discouraging the use of new products or interventions. For example, providers report they have concerns about community backlash, or ambivalence about adolescent girls taking PrEP without their parents’ knowledge. They also report the need to balance concerns about adherence with the level of risk. The findings offer insights that can be applied to provider training and support. This report from Zimbabwe, and OPTIONS’ other KAP studies in Kenya and South Africa, may well be relevant to other places trying to enhance provider skills as part of the rollout of PrEP.
- PrEP Costing Guidelines lay out the elements of estimating the cost of PrEP and how to adapt them appropriately and transparently for different objectives. These guidelines are written for individuals whose task it is to collect, evaluate and utilize cost data, and who may have differing levels of familiarity with economics.
- Common Agenda for the Dapivirine Ring is for stakeholders working on aspects of planning for the introduction of the dapivirine vaginal ring, and summarizes key components for ring introduction, lists ongoing and planned efforts, and proposes next steps for streamlined and coordinated rollout.
- The Dapivirine Ring Introduction Matrix shows the findings from a pilot discussion in Zimbabwe that explored how to integrate a new prevention product such as the ring into existing programs already focused on oral PrEP. The pilot discussion included policy makers, regulators, researchers, implementers and other partners. This resource explores where existing capacity could also support the rollout of the ring, and it suggests where additional planning and assistance would be needed. The matrix also highlights how ring introduction can strengthen other prevention efforts, especially oral PrEP.
These and other tools being developed by the OPTIONS Consortium (which is co-led by FHI360, AVAC and WITS RHI) can be used in the planning for future HIV prevention products as well. The OPTIONS Consortium is a major contributor to the growing body of technical resources for delivering PrEP options at scale in key countries, and complements work being done by many other efforts and partners, including the Prevention Market Manager project that looks to accelerate PrEP access and also improve the introduction of prevention options still in the research and development pipeline. These efforts dovetail with AVAC’s advocacy programs to close the multi-year gap from proven efficacy to access that has stalled the delivery of prevention in the real world.
Taken together this body of work found on PrEPWatch.org, can serve as case studies and templates to help implementers work swiftly, improve the quality of the delivery of prevention products and maximize their impact.
ECHO Trial Results Released: Advocate’s alert
Stay tuned for more updates, and find the latest at www.avac.org/echotrial.
Today at a satellite symposium at the South African AIDS Conference linked to a publication in The Lancet, the ECHO trial of contraceptive use and impact on HIV risk released its results. The Evidence for Contraceptive Options and HIV Outcomes Study, or ECHO, was designed to evaluate the risk of acquiring HIV in HIV-negative women who used the copper intrauterine device (Cu-IUD), a levonorgestrel (LNG) implant (Jadelle) and depot medroxyprogesterone acetate-intramuscular (DMPA-IM), also known as Depo-Provera.
The topline finding: There was no substantial difference in HIV risk among women using DMPA-IM, the LNG implant or the copper IUD. These are long-awaited data from the most rigorous trial of HIV and contraceptive interactions in history. They are an urgent call to action at a time when women’s reproductive health and rights are under threat in many countries, and the mobilization by and for women’s lives is vibrant and strong.
As AVAC and the women advocates who have led this work in Africa have said in the months and years prior to the result: ECHO must prompt action. Now is the time for investment in woman-centered programs that offer a full range of contraceptive choices and HIV prevention strategies at the same site and in the context of a true informed-choice approach. The ECHO results tell us this is the case. The women who made the trial possible deserve nothing less.
Find the AVAC press release here and commentary from Yvette Raphael in an op-ed on the results in the South African Mail & Guardian’s Bhekisisa health journalism center. And we hope you’ll read on and join us in the fight!
This update provides:
- Basics on the data release
- What are the topline trial findings?
- What do the results mean – including core messages coming from the HC-HIV Civil Society Advocacy Working Group, the Africa-based women’s network led by ICW EA, with AVAC
- Who should act—and how?
- Trial background
ECHO Data: The basics
What are the topline the trial findings?
The ECHO trial did not find any significant difference in HIV risk among women using the three methods studied: DMPA-IM, LNG implant and the copper IUD. Not very many women used pre-exposure prophylaxis, or PrEP, for HIV prevention during the trial; women who used DMPA-IM reported more condom use and fewer partners. These choices don’t seem to have made a difference in HIV risk.
All of the contraceptive methods tested were safe, effective and acceptable; the majority of women stayed on the method that they were assigned to use. Very few became pregnant while they were using the method.
There were high HIV incidence rates in all three arms of the trial. This does not mean that the methods increased women’s risk. These incidence rates are comparable to those seen in young women in these countries in other trials and contexts. What is notable, though, is that many trials with comparable incidence rates recruited women with specific HIV risk factors, such as numbers of partners, commercial sex work, sexually transmitted infections, etc. In ECHO, HIV risk factors were not part of enrollment criteria. The participants were sexually active young women looking for contraception. ECHO gives a stark picture of the risk facing these young women. HIV prevention services must meet them where they are—in contraceptive clinics and other related services.
What do the results mean?
The results are a clear call for contraceptive programs that offer more method choices, including DMPA-IM for women who want to start or continue it, along with comprehensive HIV prevention interventions. The new information from ECHO should be used to improve counseling, expand method choices and rapidly and urgently integrate HIV prevention and treatment with contraceptive programs. The level of HIV risk among eSwatini, Kenyan, South African and Zambian women in the trial was profoundly high. The majority of the participants were under 25, who were not identified as at high risk for HIV—but were simply sexually active and seeking contraception.
The HC-HIV Civil Society Working Group says:
The ECHO results are not “good news”. The women in the trial did not have any specific HIV risk criteria. They recruited women who wanted contraception and were sexually active. It is a wake-up call to put HIV prevention on site at every family planning clinic including PrEP and female condoms with peer support, trained providers.
A key question about DMPA-IM has been answered, but that does not mean the method can continue to dominate women’s contraceptive programs in East and Southern Africa. We don’t believe that DMPA-IM should continue to be the only long-acting method available. Black and brown women in East and Southern Africa want choices, dislike side effects and deserve equity with the high-quality contraceptive programs often available in high-income countries.
ECHO shows method mix is possible. Women use many things. Make it happen.
Women need strategies to prevent pregnancies and HIV infection at the same sites, from the same providers, in a rights-based, woman-centered context. Throughout ECHO, the risks of unplanned pregnancy and HIV were pitted against each other by scientists and normative agencies. Now is the time for integration. This has to include investigation—more research on how to deliver services that meet contraceptive and HIV needs well, what is driving HIV risk and how to address it, and more.
Click here for the full statement.
Who should act—and how?
- WHO should follow through on its commitment to rapidly convene a Guidelines Review Group (GRG), issuing a clarifying statement for countries in the interim. This GRG should include African women who have led advocacy on this effort for nearly a decade.
- Every east and southern African country must now make or implement, with full funding, a plan—with milestones—for expanding contraceptive method mix and uptake, and integrating HIV prevention into contraceptive service points.
- The upcoming WHO meeting in Zambia prompted by the ECHO results should generate a declaration of commitment to this, along with a commitment from funders to put money into this work and revisit the key milestones across the regions and in countries in one year’s time. This review could be guided by the method mix and choice indicators developed by FP2020 and the integration index piloted by the US group CHANGE.
- This review must be validated by “ground forces”—women who live and work and love in the places where this trial happened. There is nothing for us without us, nothing that can call itself a “woman-centered approach” with a straight face if it does not have women, especially young women, in the lead.
Trial background
The Evidence for Contraceptive Options and HIV Outcomes Study, or ECHO, was designed to evaluate the risk of acquiring HIV in HIV-negative women who used the copper intrauterine device (Cu-IUD), a levonorgestrel (LNG) implant (Jadelle) and depot medroxyprogesterone acetate-intramuscular (DMPA-IM), also known as Depo-Provera. The trial also compared pregnancy rates among women using these methods, documented rates of method discontinuation and switching, and provides a valuable body of evidence about acceptability of these methods among African women.
Many women1 at risk for HIV are also concerned about avoiding or postponing pregnancy. Some observational studies have suggested that specific injectable contraceptives (e.g., DMPA-IM)2 can increase women’s risk of acquiring HIV, while other studies have not suggested this link between DMPA-IM and HIV risk. Before ECHO, very little was known about other methods and their relationship to HIV risk—no other randomized trial had been conducted on the relationship between HIV risk and a contraceptive method. ECHO was designed to gather high-quality information about how different methods affected risk—whether increasing or possibly decreasing it. One key goal for the trial was to gather information that could be used to shape the WHO classification of and, by extension, the service-delivery approaches for the three methods. In the past years, WHO has used its Medical Eligibility Criteria system for evaluating contraceptives to signal the theoretical possibility that DMPA and similar methods might increase HIV risk. This complex classification hasn’t translated into action in terms of women being informed about risks and benefits, or into procurement of additional alternative methods in most settings. ECHO was also designed to help understand acceptability of methods not widely used in the trial countries.
1 Throughout this document, “women and girls” refers specifically and exclusively to cisgender women and girls in all their diversities. Data on transgender women, hormonal contraception and HIV risk are not available.
2 The World Health Organization (WHO) identifies this “theoretical or possible risk” in its current classification of three products: DMPA-IM, NET-EN (another injectable that uses a different hormone from DMPA) and DMPA-Subcutaneous, also known as DPMA-SC and Sayana Press, which contains the same hormone as DMPA-IM but uses a different, simpler injectable delivery method.
ECHO results coming Thursday; here are opportunities to engage
The results of the ECHO Study (Evidence for Contraceptive Options in HIV Outcomes)—a trial designed look at whether three specific contraceptive methods (DMPA-IM, the Jadelle Implant and the Copper IUD) impact women’s HIV risk—will be announced on Thursday, June 13, 14:00–15:30 SAST / 8:00–9:30am EDT at the South Africa AIDS Conference (SA AIDS) in Durban.
Over the coming days and weeks there will be a number of opportunities to learn more, discuss the data and work with fellow advocates on what’s next. Read on for details:
- The results announcement session at SA AIDS will be livestreamed on Thursday, June 13 at 14:00–15:30 SAST / 8:00–9:30am EDT.
- Register for a webinar, A Roadmap for Results: Understanding the ECHO Study Results, which will be held following the results announcement (June 13, 17:00 SAST). Hosted by AVAC and FP2020, this webinar will explain the trial, provide topline results, outline next steps, and offer key advocacy messages to help all stakeholders understand the findings.
- Check out the newest episode of our Px Pulse podcast, which features our interview with two leaders from the ECHO team, Jared Baeten and Helen Rees. They talk about what the trial can and cannot tell us. And you’ll hear leading women’s advocates from several countries where the ECHO study took place share their demands as the ECHO trial raises the volume on an urgent conversation—how to empower African women around comprehensive sexual and reproductive health and rights.
- The week of June 17, AVAC and partners will host a webinar for advocates to look beyond the trial and shape an advocacy agenda for a women-centered, women-led future of informed choice and integration of HIV services and sexual and reproductive health and rights.
The ECHO Study randomly assigned HIV-negative women from eSwatini, Kenya South Africa, Zambia to use one of three contraceptive methods—the copper intrauterine device, the Jadelle implant, and DMPA-IM, also known as Depo. Women received counseling, HIV prevention and PrEP referrals where available. The data will show rates of new HIV infections among the three groups; the study is designed to see if any of the three methods impact women’s HIV risk. A key impetus for the trial was ongoing uncertainty about whether DMPA impacts women’s risk of HIV.
Stay tuned for additional information and advocacy opportunities as the story unfolds!
Don’t Miss This Week’s Webinars
[UPDATE: Slides and recordings from both webinars are now available. Links are provided below.]
This week, AVAC is hosting two webinars, each bringing focus to different challenges facing HIV prevention right now.
First up is Tuesday’s webinar, May 21, 9-10:30 EDT, Breaking the Cycle of Transmission: Increasing uptake and effective use of HIV prevention among high-risk adolescent girls and young women in South Africa—quantitative findings & segmentation. Recording available.
Presented by the HIV Prevention Market Manager, this webinar is the second in a series presenting findings from research on what encourages or discourages the effective use of HIV prevention among adolescent girls and young women. Check out the first webinar in the multi-part series, which covered the qualitative results.
Next up, on Thursday, May 23, 9-10am EDT is The Growing Epidemic of Vaccine Hesitancy and the Implications for Global Health. Recording available.
Join us to hear Heidi Larson, the Director of The Vaccine Confidence Project at the London School of Tropical Medicine and Hygiene discuss what is called vaccine hesitancy and its implications across global health. We’ll also be joined by Laura Lopez Gonzalez, deputy editor at Bhekisisa, a health journalism center of the Mail & Guardian newspaper in South Africa. They’ll share perspectives on broader vaccine issues that impact the AIDS response as they play out in the media.
Bring your questions and join the conversation!
And if you missed it, check out last week’s HVAD webinar on HIV vaccine science and advocacy priorities. Download the slides and recording here.
Will a Vaccine Crisis of Confidence Impact the Global Response to HIV?
This HIV Vaccine Awareness Day, AVAC has an updated toolkit of resources for translating HIV vaccine research with a renewed sense of urgency, and two dedicated hashtags to rally the call on social media: #HIVvaccineAware and #HVAD2019. We hope you’ll join the conversation — with the updated HVAD 2019 toolkit and our upcoming webinars!
Mitchell Warren is Executive Director of AVAC. This post first appeared in Science Speaks.
This year’s annual HIV Vaccine Awareness Day arrives Saturday at a promising and also perilous time for vaccines.
On one hand, multiple vaccine candidates that might protect against HIV are advancing in large clinical trials. Thanks to an extraordinary global commitment and US government investment, two large-scale studies testing different HIV vaccines are underway in Africa, and a third trial is slated to start soon in the Americas and Europe. Simultaneously, researchers are advancing other promising approaches in the lab, such as those that harness powerful anti-HIV antibodies to protect against the virus.
This golden age of vaccines is not limited to HIV. Infants in three African countries will soon receive a vaccine designed to reduce malaria — a disease that kills one child every 30 seconds, according to UNICEF. A vaccine to prevent pulmonary tuberculosis in adults may soon advance to a large-scale trial and could become a central component of strategies to contain this life-threatening disease that, when left untreated, kills half the patients it affects. And an experimental Ebola vaccine is being deployed with urgency to stop a growing outbreak in the Democratic Republic of the Congo.
Vaccines have changed our lives in fundamental ways. In many parts of the world, vaccine-preventable diseases that terrified our parents and grandparents are dim memories today. But vaccines are not simple products. Many have challenges. Some are hard to manufacture; some only provide temporary or partial protection. Improving vaccines, using them effectively, educating potential users and ensuring that vaccines reach those in need all require public support and engagement.
Today, however, a rising global tide of anti-vaccine misinformation and sentiment is challenging that informed engagement — with potentially deadly results. Anti-vaccine fervor, based on discredited pseudo-science and too often endorsed by religious, government or community leaders, has led to the resurgence of diseases, such as the current — and completely preventable — measles outbreak, that were once considered conquered.
The implications of the anti-vaccination movement are enormous. Just as vaccines against some of the greatest global killers finally come within reach, fear and misinformation could diminish vital commitments to continued vaccine research, and important investments in vaccine education and delivery. Effective vaccines might not get developed, manufactured, distributed or used due to misguided, anti-vaccine sentiment — potentially putting millions of people at risk for entirely preventable deaths and diseases.
In the case of HIV, a faltering commitment to a vaccine would be devastating. Even with highly effective treatment and a daily prevention pill (known as PrEP) that can reduce HIV infections by more than 95%, nearly 2 million people still become infected with HIV each year. In the United States, a government pledge to end the domestic epidemic must confront the reality of an HIV infection rate that has barely changed since 2013. And in many parts of the world, new HIV infection rates, especially among vulnerable and marginalized populations, are actually rising rather than falling. All of these statistics point to one undeniable truth: we have made important progress against HIV, but this epidemic will not end without a vaccine.
In labs around the world, vaccine researchers are doing what we’ve asked them to do…making unprecedented progress against some of the most complex pathogens ever targeted.
Now, we must all leverage that scientific progress to rebuild a vital component of public health – faith in and support for vaccines. New vaccines in development present the greatest opportunity to save lives in human history. We cannot allow that opportunity slip away due to indifference, neglect or misinformation.
This HIV Vaccine Awareness Day, it’s time for a renewed global commitment to vaccine research, development and delivery. Vaccines save lives today, and a new generation of vaccines can save even more lives tomorrow.
HVAD 2019: Vaccine science needs your support!
[UPDATE: Slides and recordings from both webinars are now available. Links are provided below.]
HIV Vaccine Awareness Day (HVAD) 2019, on May 18, comes with promising headlines about advances in potential vaccines for HIV and other diseases that imperil public health. But, 2019 has also seen outbreaks of a highly infectious, vaccine-preventable disease, the result of misinformation and fear being spread by anti-vaccine campaigners.
This HVAD, AVAC has an updated toolkit of resources for translating HIV vaccine research with a renewed sense of urgency, and two dedicated hashtags to rally the call on social media: #HIVvaccineAware and #HVAD2019. We hope you’ll join the conversation — with the updated HVAD 2019 toolkit and our upcoming webinars (below and online)!
Explore all of our updated HVAD resources:
- HIV Vaccines—Key Messages lays out advocacy priorities and important updates from the field for the year ahead.
- HIV Vaccines, An Introductory Factsheet provides basic information on concepts and trials in vaccine research.
- Vaccines 101 slide deck reviews basic concepts and the status of research and development.
- HIV Vaccine R&D in 2019 slide deck provides this year’s updates on the state of the science and clinical trials.
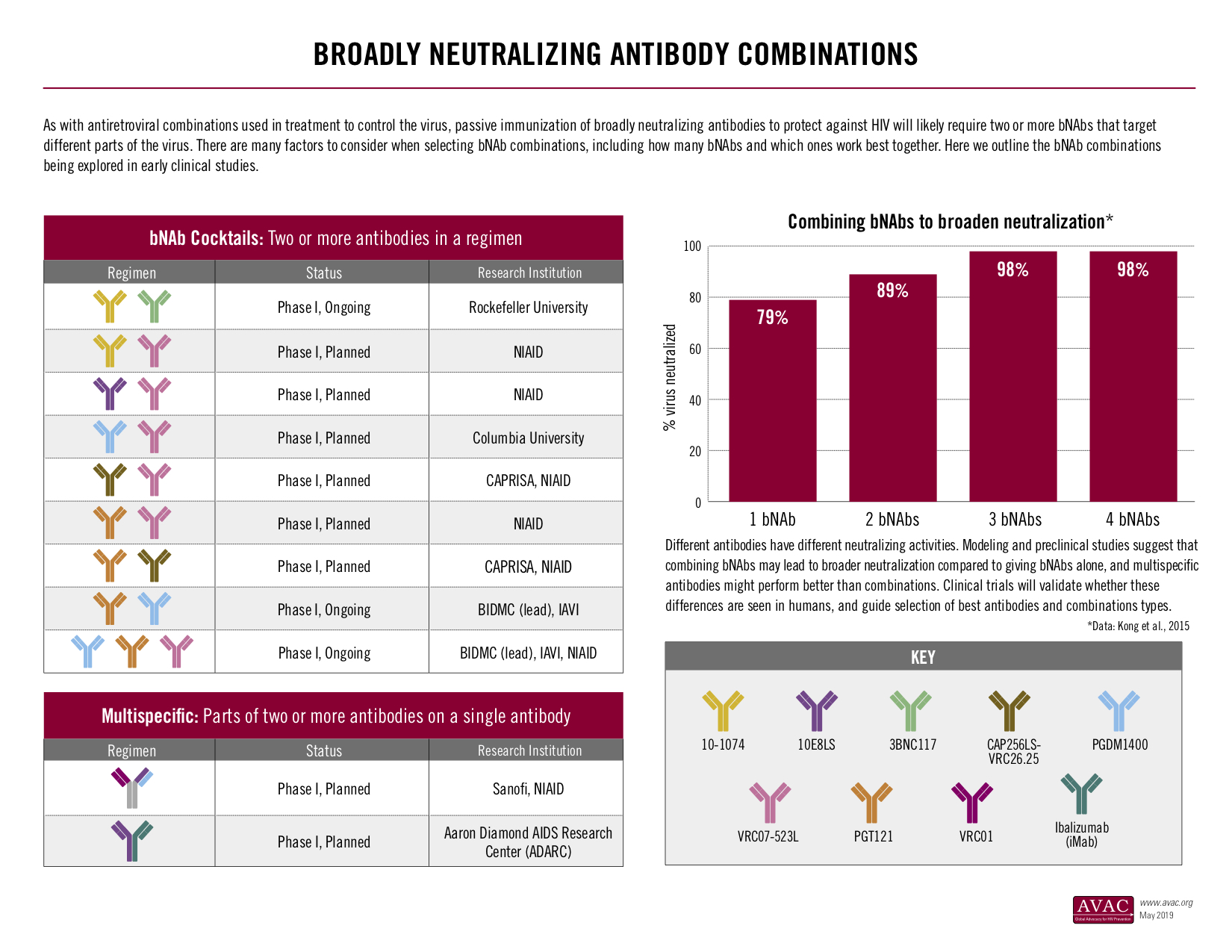
- A suite of infographics, including updates on the Vaccine Efficacy Trials Pipeline; HIV-Specific Neutralizing Antibodies: Targets and research status; HIV Vaccine Trial Participation in 2019 and others that provide a visual overview of the HIV vaccine research landscape.
One of AVAC’s HVAD Toolkit infographics showing bNAb combination research.
We also hope you will join two upcoming webinars:
- On May 16, Mary Marovich, the Director of the Vaccine Research Program at the Division of AIDS at the National Institute of Allergy and Infectious Diseases, and long-time HIV vaccine research advocate and community leader, Mark Hubbard, discussed the current landscape of vaccine research. Recording: YouTube / Audio / Introductory Slides / Mary Marovich’s Slides
- On Thursday May 23, 9am ET, Heidi Larson, Director of the Vaccine Confidence Project will discuss the implications of vaccine hesitancy and other issues along with Laura Lopez Gonzalez, deputy editor of South Africa’s Bhekisisa Health Journalism Centre.Recording: YouTube / Audio / Slides
Resources like these are essential for public understanding and support for vaccine research. Vaccines are not simple products. They require sustained investment to develop, they can be challenging to manufacture, and just as challenging to explain to potential users.
This HVAD, join us in thanking the ongoing dedication and ingenuity – of scientists, trial participants and community advocates – to find a vaccine against HIV, and let us renew our commitment to advance public understanding and support for vaccine research, development and delivery.
Zambia CSOs on the Frontlines of Key Population Planning in Zambia
In early May, AVAC joined Zambian civil society organizations (CSOs) in Lusaka for a kick-off meeting to plan the country’s Key Population Investment Fund (KPIF) activities. The lessons learned there are highly relevant to civil society in every country where KPIF will be introduced. This is time sensitive and urgent for all of us who want to see this investment have a meaningful impact.
Background
Two years ago, at the United Nations’ 2016 High-Level Meeting on Ending AIDS, the world came together to plan how to achieve the Fast Track goals aimed at radically reducing AIDS deaths and new infections. A few member states, like Iran, pushed back against the bold new initiatives needed to reach and serve key populations in order to achieve those goals. Civil society raised its voice in the halls and in rain-soaked protests to call for approaches that placed services for and led by key populations at the center of the response. Responding to this call, PEPFAR’s Ambassador Debbi Birx announced a new $100 million investment in the KPIF. This investment, modeled on the DREAMS Innovation Challenge, hit a number of delays in its implementation. Now that it has finally been launched, some of the details about its structure are clearer: these PEPFAR grants will be made through USAID and CDC to implementing partners in each country, who then should be directing resources to local key population (KP)-led organizations.
As our experience in Zambia shows, this investment in actual KP-led organizations that have the trust and faith of the community needs to be tracked and made a non-negotiable in every country. The program’s impact will be measured via the Monitoring, Evaluation, and Reporting Indicators that PEPFAR uses to monitor all partners’ performance. Since these have been largely focused on large service delivery programs – testing, linkage to treatment, PrEP enrollment – it can look like a barrier to eligibility if the local KP-led groups do not provide services. However, direct service delivery is not a requirement for receiving KPIF funds. This is another point to emphasize and track as KPIF rolls out.
What happened in Zambia?
AVAC, MPact, Health GAP and many other international groups have worked alongside African KP organizations to demand that the KPIF money flow to groups that know what needs to be done and have the trust of the community. At AIDS 2018, these groups called upon PEPFAR to create an independent KP advisory group for the fund, and they continued to question the funds structure, timelines and purpose at the PEPFAR Regional Planning Meetings in early 2019.
In May, AVAC joined Zambian KP groups and the CDC for the national KPIF planning process. AVAC and these CSOs met separately the day before the meeting to plan demands and review CDC’s proposed approach for implementation, which had been developed without engaging civil society.
Faced with this information, the CSOs decided it was strategic that they speak in one voice and this led to one of the most inspiring moments of the week. A new and entirely unprecedented consortium of Zambian KP CSOs was formed. The Zambian Key Population Consortium (Zam-KPC) aims to push forward an advocacy agenda focused on human rights and HIV prevention, treatment and care, and preventing discrimination and abuses faced by key populations. For far too long, Zambia’s key populations have been left behind in terms of direct engagement on issues that affect them. With this coalition, Zam-KPC intends to not only have a seat at the table but to monitor and implement programs that target KP communities.
Zambian CSOs came up with their own demands for how the KPIF funds should be invested and how civil society should be engaged moving forward:
- No implementation to begin without an identified KP CSO sub-award partner.
- Work with KP groups to develop sub-awards and build technical capacity of KP CSOs.
- Work with CDC Zambia to support the structural development of the KP Consortium.
- Use available funds from PEPFAR’s Zambia-focused country operational plans (COPs) to build capacity in CSOs so they can provide prevention services including PrEP, distribute HIV self-test kits, and understand PEPFAR’s requirements for monitoring, evaluation and reporting (MER indicators).
CDC and their lead implementation partner, Centre for Infectious Disease Research in Zambia (CIDRZ), agreed to all of these demands, and further committed that this work would happen in the next five months before any sub-grants are made to KP organizations.
Next Steps
The next five months are critical to the Zambian KPIF investment. AVAC will continue to provide support to the Zambia KP Consortium in making sure they are holding themselves, CDC and CIDRZ accountable. We will be actively monitoring the same process in other countries and supporting activists in those countries to get involved.
Lessons Learned
We all have to pay attention to how these KPIF funds are moving in country. Here are some of our emerging lessons learned:
- Meet with the funding agencies (CDC and USAID) and their KPIF implementing partner (IP) as soon as possible—ask for updates on the IPs plans for geography, KP partners and roles. No decision is set until KPs sign off. Now is the time to engage. If you don’t know who your agency is or what the schedule is, email [email protected].
- Build a bold, shared agenda. PEPFAR has, in the past, critiqued CSOs for being divided or in competition. Now is the time to work in solidarity—the biggest wins will come from a strong coalition of groups. If there is no KP coalition, you may want to build one; in most countries, KPIF funds can support the work building coalition.
- Consortiums do not have to apply for KPIF funds but member organizations should apply based on needs and strengths. For example, consortiums can work to identify which member is best placed to deliver particular services and decide that they should be the one applying for that portion of the programming.
- Advocacy and activism improve uptake and service delivery. KP groups do not need to be service providers to get these resources, but they do need to understand how the results will be evaluated based on MER indicators.