ARV-based HIV prevention implementation is on a roll, with WHO recommending daily oral PrEP as an option for all people at substantial risk of HIV acquisition—and also calling for immediate offer of treatment to all people living with HIV. TDF/FTC (brand-name Truvada) is now approved for HIV prevention as PrEP in France, Kenya, South Africa and the United States—with more countries sure to follow in 2016.
These advances may lead people to ask whether the world still needs an AIDS vaccine as part of the strategy for ending the epidemic? And will a vaccine be needed in five or ten years time—the likely timeframe for results on today’s leading candidates to end the epidemic? For prevention advocates who don’t want to settle for a limited set of options, and who understand the potential revolutionary impact of a vaccine, a new modeling analysis, published this month in the open-access journal PLoS ONE, provides some clear answers to this question.
The paper, Exploring the Potential Health Impact and Cost-Effectiveness of AIDS Vaccine within a Comprehensive HIV/AIDS Response in Low- and Middle-Income Countries, was authored by IAVI, Avenir Health and AVAC, with financial support from USAID.
It looks at the potential impact of an effective AIDS vaccine in the context of expanded coverage of early treatment, PrEP and other existing strategies reflected in the UNAIDS Investment Framework Enhanced (IFE). The IFE, published in 2014, assumed scale-up of ART according to WHO 2013 guidelines along with VMMC and the potential introduction of PrEP, and an AIDS vaccine. [Note: the 2013 WHO guidelines indicated treatment for individuals with a CD4 count of 500 or less; the updated guidelines released in 2015 indicate offering treatment to all HIV-positive individuals, regardless of CD4 count.]
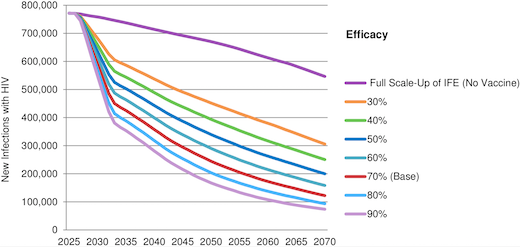
The paper reflects that if UNAIDS IFE goals were fully achieved, new annual HIV infections in LMICs would decline
from 2.0 million in 2014 to 550,000 in 2070. A 70 percent efficacious vaccine introduced in 2027 with three doses, strong uptake and five years of protection would reduce annual new infections by 44 percent over the first decade, by 65 percent the first 25 years and by 78 percent to 122,000 in 2070. Vaccine impact would be much greater if the assumptions in UNAIDS IFE were not fully achieved. An AIDS vaccine would be cost-effective within a wide range of scenarios.
This paper suggests that even a modestly effective vaccine would reduce infections significantly and be cost-effective, even as other interventions are broadly implemented. This confirms what AVAC and others have often said before: no single option will or can end the epidemic.
