The first supplies of injectable cabotegravir for programmatic use (as opposed to use in implementation studies) began to arrive in countries in 2024. Currently, 16 countries are rolling out CAB for programmatic use, with the majority of supply provided by PEPFAR, and some additional quantities procured by the Global Fund.
Source of Programmatic Cabotegravir for PrEP Supply
The HIV Prevention Pipeline
This graphic shows currently available options for HIV prevention, newly approved and recommended treatment, and those in development.
PxWire Volume 15, Issue 4
This issue provides a range of maps to help orient the field on critical HIV prevention activities: the status of delivering injectable cabotegravir (CAB for PrEP); funders and countries on track for early introduction of injectable lenacapavir (LEN for PrEP); and where new Phase 3 efficacy trials testing MK-8527 as a monthly pill for PrEP are taking place.
Read below or download a PDF version of this issue.
Progress in PrEP Uptake
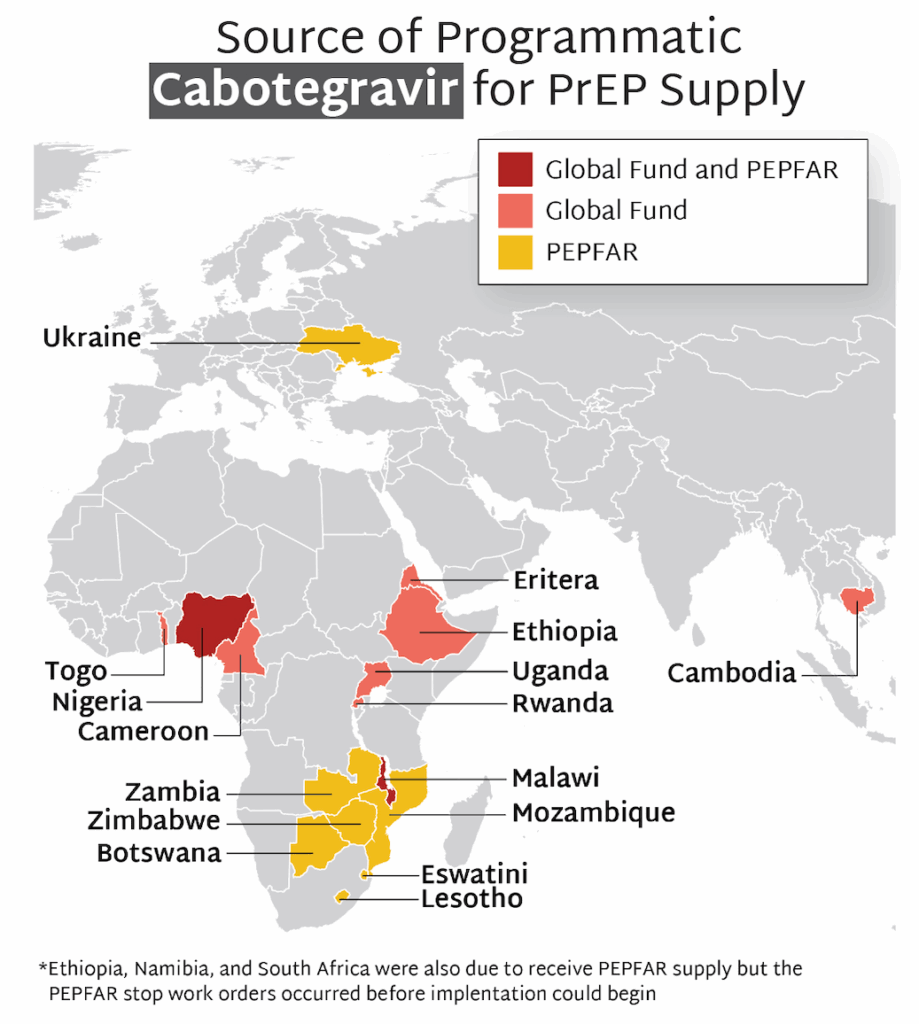
- The first supplies of CAB for programmatic use (as opposed to use in implementation studies) began to arrive in countries in 2024.
- Currently, 16 countries are rolling out CAB for programmatic use, with the majority of supply provided by PEPFAR, and some additional quantities procured by the Global Fund.
- Stop work orders for PEPFAR programming, issued in January 2025 by the new US administration, led to the cancellation of planned programs to offer CAB before they could start in Namibia and South Africa. Ethiopia’s CAB program was able to move forward with supplies supported by Global Fund.
- Stop work orders disrupted ongoing CAB programs in other PEPFAR supported countries, too. They were able to resume, though in some cases at a reduced level. Global Fund supported programs were unaffected by stop work orders and are ongoing.
- The future of PEPFAR and Global Fund supported CAB programs remains uncertain in the context of multiple forms of injectable PrEP entering the market. But lessons and insights being gathered now on delivery of injectable PrEP via these programs can be leveraged to help countries prepare for the introduction of LEN.
- In the past quarter, CAB for PrEP received additional regulatory approval in Argentina, Chile, Mexico, and Rwanda.
PrEParing for New Products
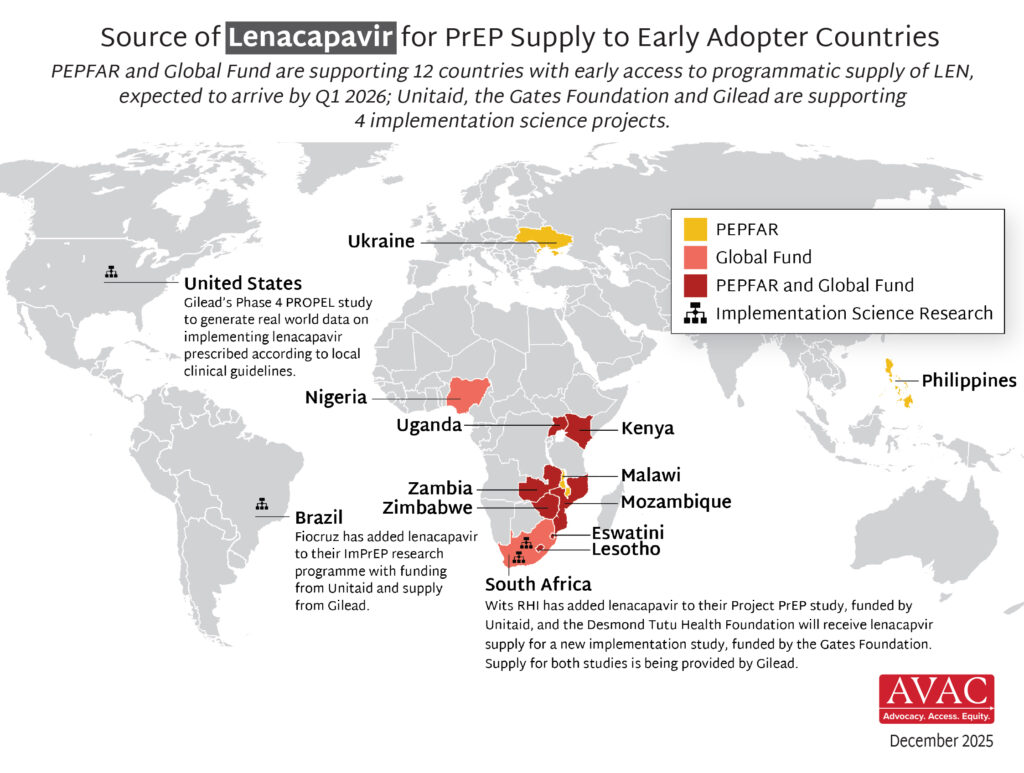
- The Global Fund, with support from CIFF, and PEPFAR have jointly committed to reaching up to two million people with LEN for PrEP over three years.
- Supply of LEN is due to begin arriving in countries in late 2025 with service delivery planned to start in early 2026.
- Ministries of Health, implementing partners and communities are now preparing for LEN introduction, and CIFF, Unitaid and PEPFAR are funding technical support efforts. PrEPWatch.org hosts updated tools to support developing guidelines, training providers, preparing delivery sites and developing demand generation campaigns.
- In addition, Unitaid, the Gates Foundation, and Gilead are supporting LEN implementation research:
- Two Unitaid-funded PrEP implementation projects – ImPrEP in Brazil (led by Fiocruz) and Project PrEP in South Africa (led by Wits RHI) – will be adding LEN.
- Gates is providing support to the Desmond Tutu Health Foundation for a new LEN implementation study, ALIGN, in South Africa.
- Gilead is funding a Phase 4 study, PROPEL, in the United States that will assess real world implementation of LEN and effectiveness outcomes.
- The supply for LEN for these projects is being provided by Gilead.
- The Elton John AIDS Foundation is providing support to Global Fund LEN programming in Kenya, Nigeria, Uganda, and South Africa.
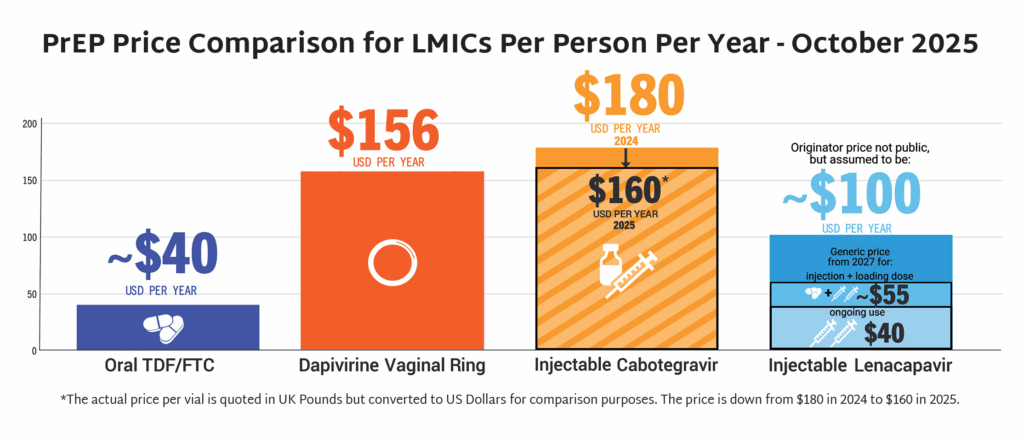
- In September, the Gates Foundation and Unitaid announced partnerships with Hetero Labs and Dr Reddy’s Laboratories, respectively, to achieve a price of $40 USD/person/year for generic injectable LEN. Generic LEN is expected to become available in 2027 in up to 120 countries.
- LEN received WHO prequalification in October, becoming the first product to be prequalified using an expedited process which took just 36 days from the date of submission.
- In the past quarter, LEN has been approved by the European Medicines Agency and submitted in Australia, Canada, and Switzerland.
The Latest R&D in the Prevention Pipeline
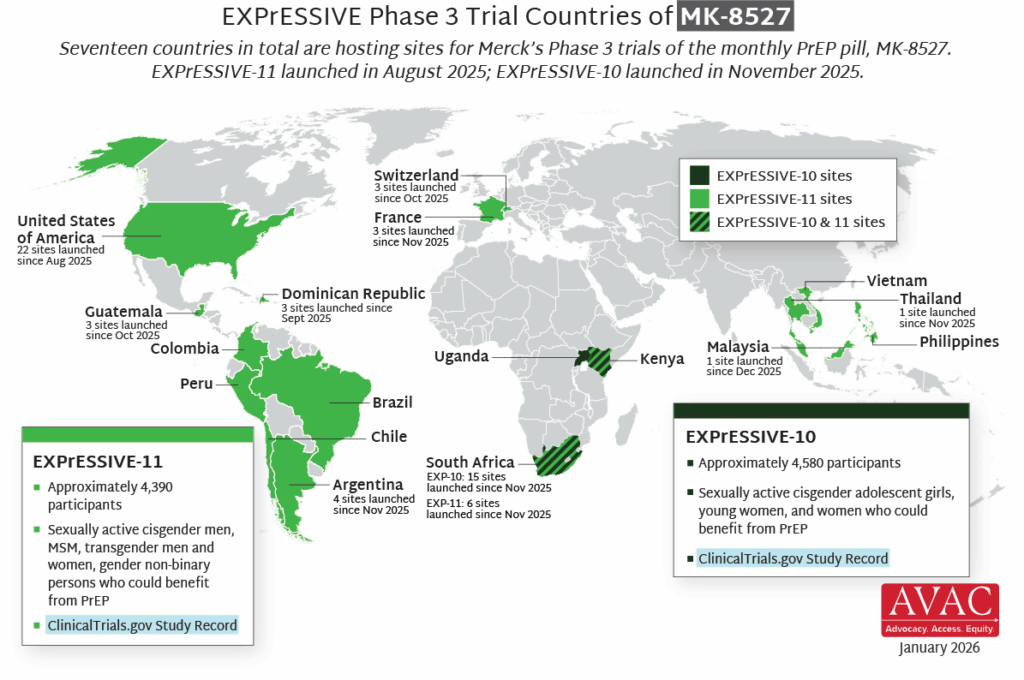
- Seventeen countries are hosting sites for the Phase 3 efficacy trials of a monthly PrEP pill, MK-8527, being developed by Merck (also known as MSD outside of the US and Canada). Merck announced the launch of the Phase 3 trials at IAS 2025 in Kigali. MK-8527 was found to be safe and well-tolerated in Phase 2 clinical trials.
- A long-acting PrEP pill would offer a unique new option to the existing range of PrEP options and could significantly expand use of HIV prevention, especially among young women, key populations, those facing stigma or access barriers, and those that prefer an oral PrEP option over an injectable.
- EXPrESSIVE-10, with funding from the Gates Foundation, is expected to launch in Q4. It will enroll about 4,600 cisgender adolescent girls, young women, and other women who could benefit from PrEP across three countries in Eastern and Southern Africa.
- EXPrESSIVE-11 launched in August 2025, and will enroll about 4,400 cisgender men, MSM, transgender men and women, and gender non-binary persons who could benefit from PrEP in 16 countries. So far, EXPrESSIVE-11 has launched at sites in Dominican Republic, Guatemala, Switzerland, and the US.
- Merck’s commitment to stakeholder engagement to date contributes an important model of Good Participatory Practice (GPP) to the field, by putting global advocates at the forefront of planning for the program and trial design. Merck has expressed a commitment to sustain this vital engagement throughout the program and next steps.
Prevention Playlist
Join
- Do Not Check That Box – Impacts From the Assault on Transgender Communities and DEI + Strategies to Sustain and Rebuild, webinar
- From Courtrooms to Communities: Funding Advocacy to Protect HIV Responses, webinar
- We Declare—Turning The People’s Declaration Demands into Actions and Accountability, webinar
- International Conference on AIDS and STIs in Africa (ICASA 2025), conference
- Subscribe to Global Health Watch
Use
- Now What with Injectable LEN for PrEP?, factsheet
- Guidelines on lenacapavir for HIV prevention and testing strategies for long-acting injectable pre-exposure prophylaxis, guidelines
- Potential Demand for LEN for PrEP, infographic
- Where We Are Now with LEN for PrEP, infographic
- PrEP Price Comparison, infographic
- HIV Prevention Product Overview, infographic
Watch/Listen
- 24 Hours to Save AIDS Research, website & videos
- New WHO Guidance on Lenacapavir for Prevention & HIV Testing for Long-Acting PrEP, webinar
- Next Up: A monthly pill for PrEP?, podcast
- PrEP Implementation — What’s worked and what are we learning, webinar
Read
- AVAC Applauds Agreements to Accelerate Market Development for Lenacapavir for PrEP, statement
- Global Advocates Welcome the Launch of Merck’s EXPrESSIVE Program, statement
- Getting Rollout Right This Time, report
- Key Messages from IAS 2025, newsletter
- Addressing Transgender Erasure in HIV Clinical Trials, publication
- Global Forecast of Long-Acting PrEP Need for Key Populations (2025–2030), report
Cabotegravir Implementation
Implementation studies completed, ongoing, or planned for cabotegravir as of October 2025. For product approvals, volumes, implementation, and price comparisons of long-acting PrEP, visit our dashboard on PrEPWatch.org.
Dapivirine Vaginal Ring Implementation
Ongoing and planned implementation studies for the dapivirine vaginal ring as of October 2025. For product approvals, volumes, implementation, and price comparisons of long-acting PrEP, visit our dashboard on PrEPWatch.org.
Cabotegravir Volume
Allocation of non-commercial cabotegravir for PrEP supply in low- and middle-income countries, 2023-2026, as of October 2025. For product approvals, volumes, implementation, and price comparisons of long-acting PrEP, visit our dashboard on PrEPWatch.org.
Dapivirine Vaginal Ring Volume
DVR supply available to low- and middle-income countries as of October 2025. For product approvals, volumes, implementation, and price comparisons of long-acting PrEP, visit our dashboard on PrEPWatch.org.
AVAC Input for Recompetition of the NIAID HIV/AIDS Clinical Trials Networks
AVAC’s formal input submitted on the re-competition of the NIAID HIV/AIDS Clinical Trials Networks. The recommendations were informed by the People’s Research Agenda (PRA), a comprehensive framework developed through consultations with over 130 community representatives across 23 countries.
Manifestations and Lived Experiences of Structural Racism for Racial and Ethnic Minority Communities Affected by HIV Across the United States
Structural racism shapes the lived experiences of racial and ethnic minority communities through political disempowerment, inequitable access to resources, and intergenerational trauma. This study provides support for the argument that the HIV and COVID-19 epidemics will not be adequately addressed without urgent action on the various ways in which structural racism manifests in the US. Given the troubling trend toward stifling discussions on race and racism, now is the time to accelerate, not subdue, national, state, and local discourse on structural racism and intersectional oppression. Now is time for bold, transformative actions.
Addressing Transgender Erasure in HIV Clinical Trials
Scorecard indicators reveal a dearth of HIV research responsive to the needs of transgender and gender-diverse (TGD) communities. The lack of TGD representation in HIV clinical trials indicates a historical erasure of TGD communities with potential public health consequences. The scorecard might guide future HIV research to be more responsive to the needs of TGD people. Published in the American Journal of Public Health.