The timeline for generic LEN for PrEP to come to market is expected to be significantly shorter than for CAB for PrEP. Bioequivalence (BE) testing for LEN, which demonstrates a generic product works in the body in the same way as the originator product, is likely to be six months, vs. the 18 months for CAB for PrEP, because of differences in the drug formulation. The rapid granting of voluntary licensing by Gilead also contributes to this shorter timeline. For the latest on LEN, visit here.
LEN Generics — Can we go faster?
Long-Acting PrEP Market Assessment for Key Populations
This market assessment supports countries, donors, implementing partners, and advocates in making informed decisions about the introduction, scale-up, and equitable delivery of long-acting PrEP among key populations and other priority groups.
The Scientific Journey of Lenacapavir — and the Urgency to Defend HIV Prevention Science
On June 11, AVAC hosted a conversation, The Scientific Journey of Lenacapavir: From basic science to clinical development to impact, to explore how US support from NIH for basic science and South Africa’s clinical research infrastructure made possible the development of lenacapavir for PrEP (LEN), a discovery in HIV prevention that went on to be named Science magazine’s 2024 Breakthrough of the Year.
As the field anticipates initial regulatory approval from the US FDA by June 19 and a WHO recommendation in July, Linda-Gail Bekker of the Desmond Tutu Health Foundation, Wes Sundquist of the University of Utah and Mitchell Warren of AVAC underscored how this moment of promise is threatened by sweeping attacks on science, research and the very systems that made the development of LEN possible.
Getting PrEP Rollout Right This Time
Thirteen years after oral PrEP’s introduction, global uptake remains slow, with just over 9 million initiations—falling short of UNAIDS’ 2025 targets. While HIV infections are declining in some regions, others are experiencing increasing epidemics. Despite challenges, lessons from oral PrEP and CAB rollout have strengthened health systems for scaling up longer-acting PrEP.
AVAC undertook a qualitative landscaping analysis to identify actionable lessons and recommendations across seven countries—Brazil, Kenya, Nigeria, South Africa, Vietnam, Zambia, and Zimbabwe—exploring themes of:
- Successes and challenges with daily oral PrEP introduction and scale-up and what can be done differently for new PrEP products.
- Public health system readiness for the introduction and scale-up of new PrEP products.
- Considerations for improving and accelerating PrEP regulatory approval, normative guidance, demand generation, stakeholder engagement, and health systems strengthening.
This analysis, conducted across seven countries before the January 2025 US foreign aid freeze, identifies urgent actions to sustain momentum. US foreign aid cuts are severely impairing PrEP delivery, jeopardizing remarkable progress made in the last 20 years. In the wake of this disruption, it is vital that efforts to mobilize and sustain an effective HIV response learn from the past and reach UNAIDS’ targets for 2030 by incorporating these insights into future planning.
Visit PrEPWatch for the full report and individual country profiles.
Protect Federal Funding for HIV, TB, and STI Research and Prevention at the National Institutes of Health
AVAC and 627 organizations, institutions, researchers, clinicians, public health advocates and stakeholders submitted a written letter to the Senate HELP Committee urging lawmakers to reject the cuts to NIH funding for HIV, TB, and STI research and highlighting the impact of these cuts on lifesaving innovation and research infrastructure.
Long-Acting PrEP Real-Time Data Dashboard
The GBGMC dashboard provides updated insights on the effectiveness of Long Acting PrEP interventions, user demographics, and access rates across regions. Visit here.
Why HIV Prevention Must Not Be Left Behind
In this presentation at the INTEREST 2025 conference, Rhoda Msiska of Copper Rose Zambia emphasizes the urgency of protecting the progress made in scaling up PrEP and the need to act now to expand access to new HIV prevention tools like injectable lenacapavir (LEN) and the Dual Prevention Pill (DPP).
Advocates’ Guide: Understanding the President’s Proposed Fiscal Year 2026 (FY26) Budget and Its Implications for Science, Research and Global Health
The US administration’s proposed Fiscal Year 2026 (FY26) budget marks a sweeping rollback of federal investment in health, research, and global development. For advocates, researchers, and implementers, this proposal demands urgent attention and action.
This initial “skinny budget” is a proposal and not yet law. A more detailed proposal will be released by mid-to-late May and the US Congress will ultimately decide actual funding levels for FY26, which begins October 1. So, advocates must speak up now to protect funding for research and programming that saves lives and livelihoods.
Here’s what advocates need to know and do:
Big Picture: A Dramatic Retrenchment
The budget proposes $163 billion in cuts to non-defense discretionary spending, including a 26% reduction to the Department of Health and Human Services (HHS)— the department that oversees the US National Institutes of Health (NIH), the Centers for Disease Control and Prevention (CDC), and the Food and Drug Administration (FDA). These cuts are completely offset by an increase to defense spending and reflect a shift toward the elimination of science and programming tied to diversity, equity, inclusion (DEI), gender, and climate, and a redirection of funding toward defense and “America First” priorities—priorities that put the perceived interests of the US and its citizens over other national and global issues.
Detailed Analysis and Implications
Health and Biomedical Research
The proposed cuts to HHS would gut federal support for health and biomedical research, dismantling key programs at NIH and CDC. They threaten progress on infectious diseases, health equity, and pandemic preparedness—undermining decades of scientific gains and leaving communities vulnerable.
NIH is Cut by $17.9 billion losing HIV and global health research
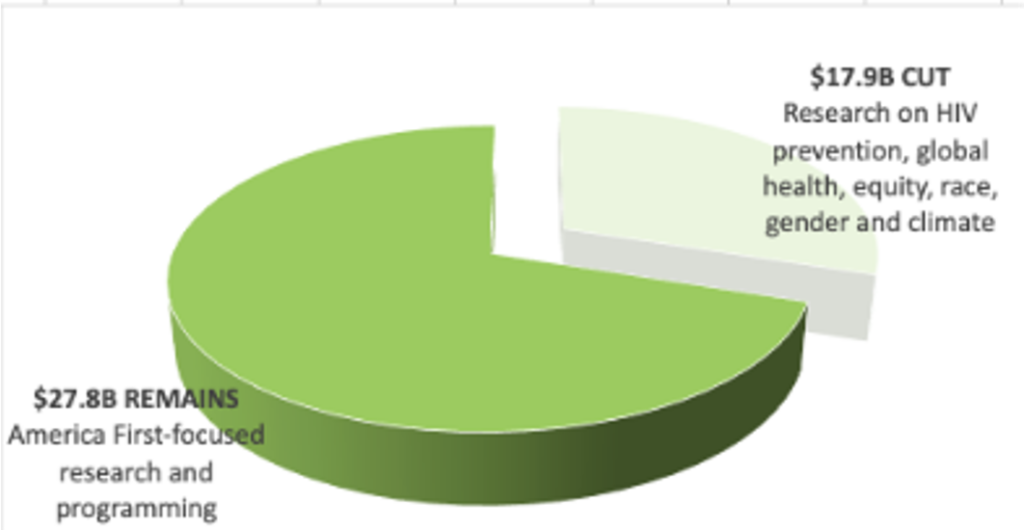
- Preserves $28 billion of the $46 billion for NIH overall, but excludes HIV prevention, global health, and health equity research.
- Reorganizes NIH into 5 “realigned” institutes, removing focus on climate, gender, racial equity.
- Eliminates the Fogarty International Center and the National Institute on Minority Health and Health Disparities.
Centers for Disease Control and Prevention (CDC): Cut by $3.59 billion
- Eliminates Global Health Center and National Centers on environmental health, injury prevention and chronic disease prevention.
- Eliminates DEI programs and shifts the burden for pandemic prevention and response.
Agency for Healthcare Research and Quality: Effectively eliminated
- Cited as redundant; targeted for work on climate and gender.
National Science Foundation (NSF): Cut by $4.9 billion (56%)
- Eliminates funding for work seen as ideologically objectionable (e.g., broadening participation and racial equity in STEM).
Global Health and Development
At a time when the USG should be expanding access to new technologies, the proposed FY26 budget guts foreign assistance funding, threatening pillars of the global HIV response: the President’s Emergency Plan for AIDS Relief (PEPFAR) and US contributions to multilateral initiatives, such as Global Fund and GAVI. The ideological targeting of family planning and gender-related programs will further weaken interventions to address HIV, which have been shown to work best within a comprehensive package of health and social services.
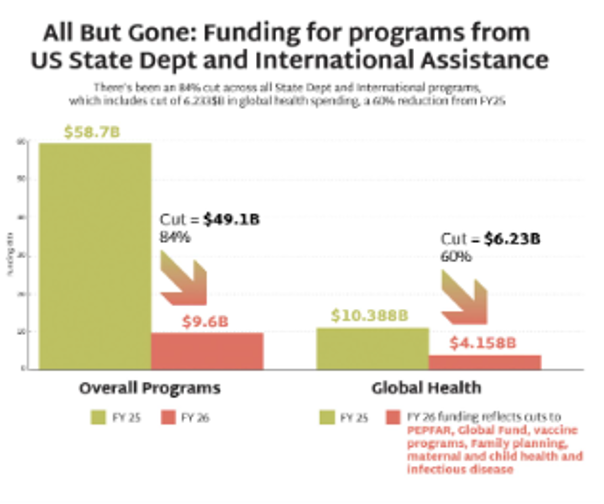
Global Health Programs: Cut by $6.23 billion
- Defunds NGOs providing family planning, impacting maternal and child health providers.
- PEPFAR preserved only for existing treatment programs and programs for the prevention of mother-to-child transmission (PMCT) , and specifically excludes primary prevention and PrEP, except for pregnant and lactating populations.
USAID Development Aid: Cut by $8.33 billion
- USAID is eliminated with the limited number of existing programs moved into the State Department.
- Eliminates DEI, climate, and gender-related programming.
- Creates new “America First Opportunity Fund” to replace foreign assistance grants with loans that prioritize US interests over humanitarian needs.
Centers for Disease Control and Prevention (CDC)
- STI, TB, hepatitis programs folded into a reduced $300 million block grant.
- For more on the specific impacts on STI programs, please read AVAC’s new brief on Worldwide Prevention, Shared Protection: Why STI Funding Matters.
Health Resources and Service Administrations (HRSA): Cut by $1.73 billion
- Ryan White HIV/AIDS Program activities not deemed core are eliminated.
Substance Abuse and Mental Health Services Administration (SAMSHA): Cut by $1.065 billion
- Eliminates harm reduction and regional substance use program grants.
Offices of Minority & Women’s Health
- Moved under a new, less visible structure.
New Initiative: “Make America Healthy Again”
- $500 million focused on lifestyle over treatment.
What This Means
- HIV Prevention R&D and global implementation is at risk. Cuts to NIH and USAID directly threaten support for clinical trials, community engagement, and biomedical innovation.
- Equity-centered research threatened. Eliminating institutes focused on minority and global health severely undermines inclusive science and jeopardizes future impact. Inclusion is not just a nice to have, it’s integral to achieving impact
- PEPFAR protections are narrow. Only existing beneficiaries are covered; scale up and innovation are excluded, compromising the imminent introduction and potential impact of injectable lenacapavir for PrEP. Funding for HIV prevention is also eliminated, except for pregnant and lactating populations.
Advocacy Priorities
- Monitor the full FY26 budget release for agency-level detail and justification.
- Engage Appropriations and other relevant Committees via coalition efforts (e.g., FAPP, GAPP, GHTC, SHF).
- Mobilize your community to contact your Senators and Representatives to let them know you oppose these cuts.
- Share your stories from researchers affected by cuts—particularly those whose work is globally focused or funded by NIH/USAID.
- Stay up to date with budget briefings and mobilization opportunities. See AVAC’s ‘Research Matters’ resource, which shares guidance and a toolkit for researchers to advocate for continued funding.
This budget is a threat to decades of progress in science, equity, and health—but it is also an opportunity to speak with clarity and urgency about what is at stake. Advocates must ensure that the future of HIV prevention, global health innovation, and equitable science is not written by politics, but by people, evidence, and impact.
Novel Study Designs for New HIV Prevention Products
A presentation by Deborah Donnell, of the Fred Hutchinson Cancer Research Center University of Washington, discussing trial design when PrEP is available. How can the field justify randomization to an experimental drug when we have something known to work? If we give study participants PrEP, how can we know a new experimental drug is working?
Modelling Shows the Potential of LA-PrEP
Modelling data from South Africa demonstrate the potential of injectable PrEP to dramatically reduce HIV incidence by up to 90% by 2044, and potentially even sooner with more aggressive uptake. This potential goes beyond South Africa, lighting the way toward epidemic control the world over. Excerpted from PxWire.