June 18, 2025
A Historic Milestone Must Now Be Matched by Urgent Action
AVAC welcomes the U.S. Food and Drug Administration (FDA) approval of injectable lenacapavir (LEN) as pre-exposure prophylaxis (PrEP). Developed by Gilead Sciences, LEN is a twice-yearly injectable PrEP option that showed nearly complete protection against HIV in the landmark PURPOSE 1 and 2 trials. Science Magazine named LEN the “Breakthrough of the Year” in 2024, a recognition that reflects its enormous potential. But that promise will only be realized if it is rolled out with speed, scale, and equity.
In our statement issued earlier today, we outline what’s needed from every stakeholder in the HIV response to meet this moment—and not squander yet another PrEP option.
As outlined in our Gears of Lenacapavir for PrEP Rollout, this moment demands unprecedented ambition, commitment, and leadership from governments, funders, implementers, policymakers, and civil society.
Advocacy Tools & Resources
Please check out all of AVAC’s advocacy tools and resources to support our collective work in advancing equitable LEN rollout as part of comprehensive, integrated and sustained HIV programs.

Getting PrEP Rollout Right This Time
Our new report examines key insights from the rollout of oral PrEP and early introduction of injectable cabotegravir and the dapivirine vaginal ring to inform a faster, smarter and more equitable introduction of future HIV prevention tools, including long-acting injectable PrEP, such as lenacapavir.
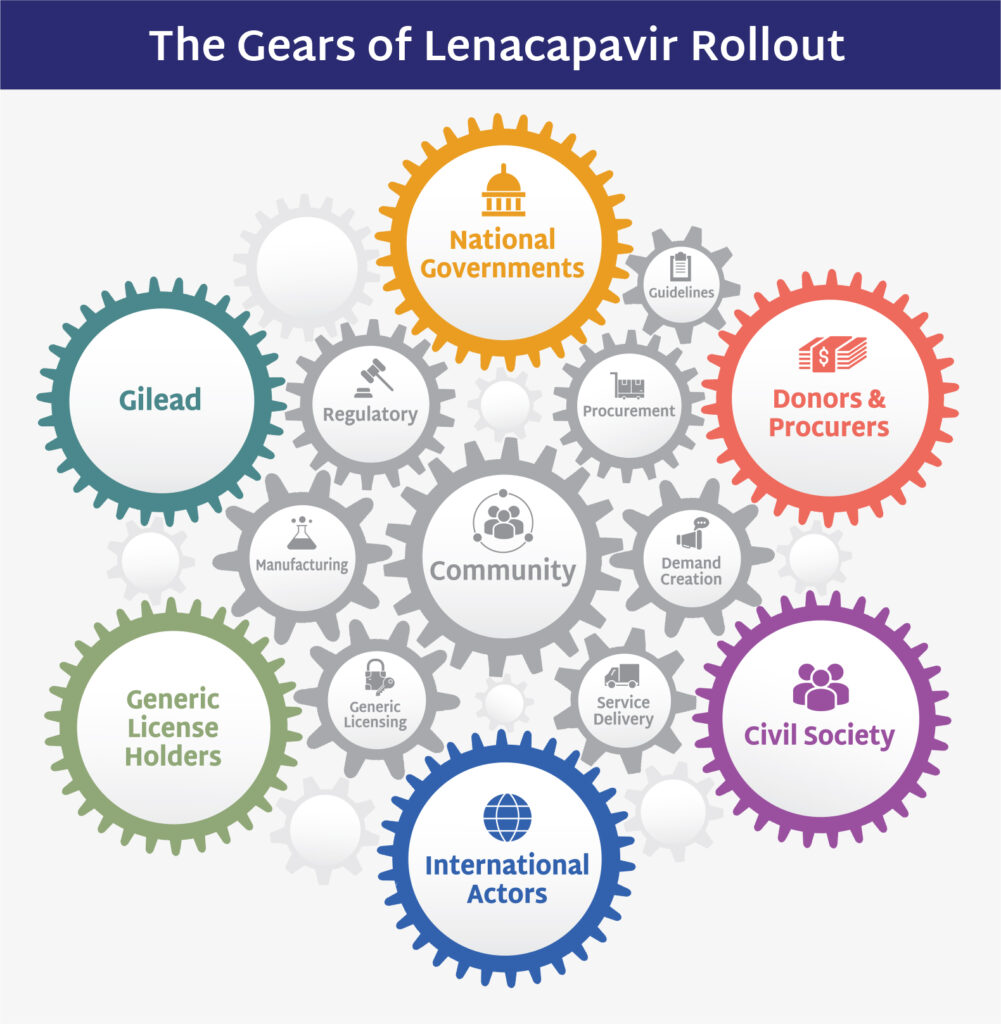
Gears of Lenacapavir for PrEP Rollout
This document outlines a focused plan for LEN for PrEP rollout over the next few years, specifying priorities by stakeholder and evaluating volume and pricing strategies.

This plan provides a broad view of all the moving parts and identifies actions and actors responsible for ensuring time is not wasted and opportunity not squandered.

The Lens on LEN: The Basics on Injectable Lenacapavir as PrEP
This advocates’ primer provides background on lenacapavir for PrEP and its trials; a summary of the early findings of PURPOSE 1 & 2; key questions and next steps.

Podcast — Lenacapavir: The case for investing in delivering HIV prevention:
This episode of PxPulse goes deep on LEN for PrEP laying out recent research findings and what they mean. And Chilufya Kasanda Hampongo of Zambia’s Treatment Advocacy and Literacy Campaign and Mitchell Warren of AVAC talk about how to change a long history of squandered opportunities to get rollout right.
Media Coverage
- Regulators Approve a Twice-Yearly Shot to Prevent HIV Infection, The New York Times
- FDA Approves Powerful HIV Drug that Nearly Eliminated Spread in Clinical Trials, NBC News
This is the moment for all stakeholders to build on the momentum science. LEN has moved faster through regulatory review than any prevention product to date. Now it’s up to all of us to make sure that that progress translates into real, sustained impact on the epidemic, led by communities around the world.
Let’s get to work.