April 11, 2024
Science and real-world experience continue to generate evidence that expanding access to PrEP options and making choice possible, must be the guidestar in HIV prevention.
Last week, NIAID Director Jeanne Marrazzo joined our Choice Agenda webinar, The More We Know: Evolving our understanding of PrEP for cisgender women, to present a re-assessment of the safety and effectiveness of PrEP options for women—including oral, vaginal ring, and injectable options. She discussed her recent publication in the Journal of the American Medical Association, HIV Pre-Exposure Prophylaxis with Emtricitabine and Tenofovir Disoproxil Fumarate Among Cisgender Women, which provides reassuring evidence that oral PrEP can reliably prevent HIV infection in cisgender women, even if it’s not taken daily. The results challenge the notion that cisgender women need to be “super-adherers” to achieve protection utilizing oral PrEP and that a one-size-fits all approach to prevention will not work.
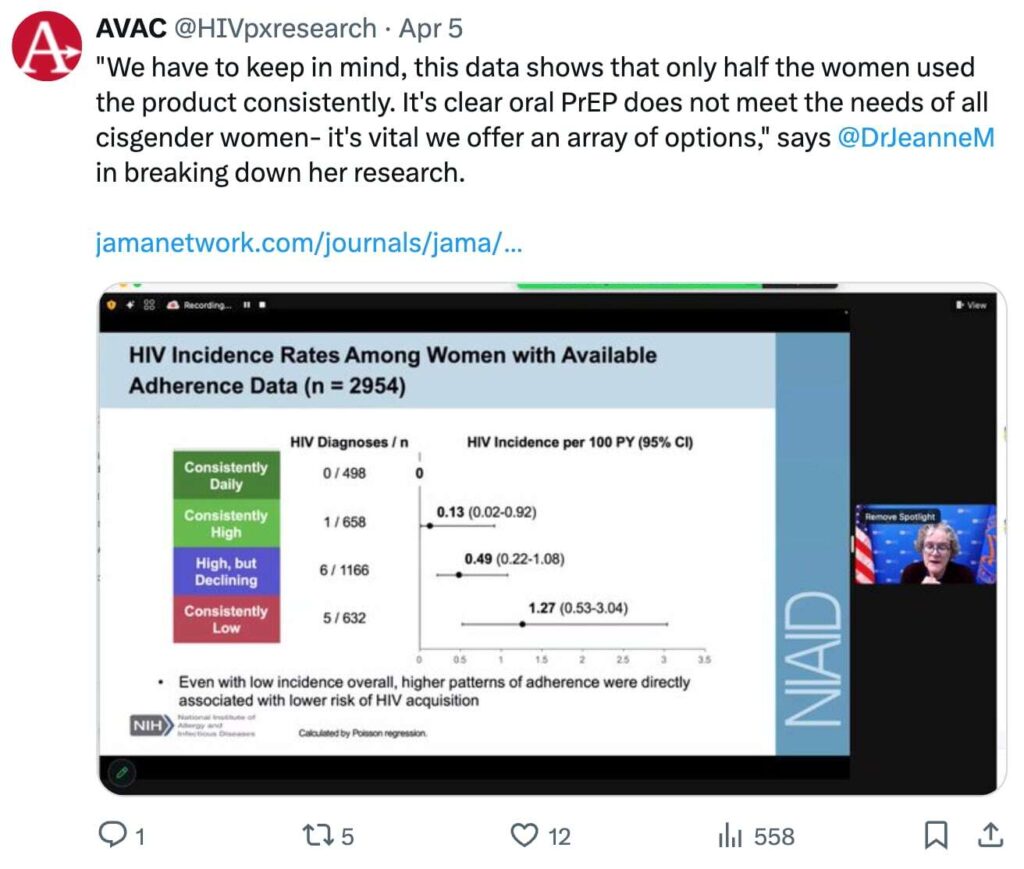
Dr. Marrazzo’s research and her comments represent a powerful voice among a chorus of champions for choice. During the same conversation, Joyce Ng’anga’a of WACI Health and the African Women’s HIV Prevention Community Accountability Board, a coalition of women and girls living and working in Africa who are united in calling for continued political and financial support for more choice in HIV prevention, updated on the recently launched Choice Manifesto. This global call to action demands investment in choice and calls for enshrining a woman’s right to choose and for African women and girls to lead the HIV response.
At the same time, PEPFAR’s Scientific Advisory Board grappled with the potential role of injectables for treatment and prevention, including an update on the current late-stage efficacy trials of injectable lenacapavir, with results anticipated later this year. It’s no secret that scale-up of all current PrEP options (daily oral, vaginal ring and injectable cabotegravir) has been slower than ideal, especially in the countries and communities with high HIV rates that need it most. So as the HIV prevention community awaits the results of the PURPOSE trials for the twice-annual lenacapavir injectable, discussing how an additional injectable option might be introduced and what infrastructure is needed to implement this new product faster than previous PrEP options is critical.
“We are 12 years since many of us gathered in Washington, D.C., at the international AIDS conference that was just two days after the FDA approved oral TDF/ FTC,” said panel moderator, AVAC Executive Director and PEPFAR SAB member, Mitchell Warren. “Twelve years later, it’s actually an abomination how poorly we have done as a global community, and when we think about equity in this country and around the world, oral PrEP is still only beginning to find its place. The dapivirine ring has struggled for a number of reasons and cabotegravir is struggling still, but I do want to highlight that it is going faster than we saw with oral PrEP, certainly in terms of regulatory approvals. And the question is how might we apply those learnings for lenacapavir in the next months and years?”
As policy, practice and budgets strive to keep up with advances in research, advocacy around choice becomes a cross-cutting priority—so that the promise of new options in HIV prevention won’t be squandered in siloed programs, or by poorly-planned supply chains, or because of disconnected policy decisions. Be sure to watch this space in 2024 as the African Women’s HIV Prevention Accountability Board, the Young Women’s HIV Prevention Council and the Global Key Populations HIV Prevention Advisory Group, amongst others, lay the groundwork to advance efforts to accelerate prevention options that people want and need.