In 2024, Gilead Sciences released findings from the PURPOSE 1 and PURPOSE 2 trials testing lenacapavir (LEN) as HIV prevention. The PURPOSE 1 trial found 100% efficacy in preventing HIV in 5,300 cisgender women in Uganda and South Africa, and the PURPOSE 2 trial showed a 96% reduction in HIV incidence among cisgender men, trans, and non-binary individuals across multiple countries. Both trials demonstrated LEN’s safety and effectiveness in reducing HIV transmission. This advocates’ primer provides background on the product and trials; a summary of the early findings of PURPOSE 1 & 2; key questions and next steps.
The Lens on LEN
Lenacapavir: The case for investing in delivering HIV prevention

The promise of long-acting PrEP has been super-charged this year by studies showing the powerful efficacy of an antiretroviral known as lenacapavir (LEN).
This episode of PxPulse goes deep on LEN for PrEP. Recorded just days before Gilead’s announcement that PURPOSE 2 also found very high efficacy, Dr. Flavia Kiweewa, a principal investigator of PURPOSE 1, the first trial to announce efficacy, lays out the research findings and what they mean. And Chilufya Kasanda Hampongo of Zambia’s Treatment Advocacy and Literacy Campaign and Mitchell Warren of AVAC talk about how to change a long history of squandered opportunities to get rollout right.
The PURPOSE1 trials announced findings in June that a twice-yearly injection of LEN was 100% effective among cisgender women, with zero new cases of HIV. And the PURPOSE 2 trial among cisgender men, and trans and non-binary people, was shown to reduce the risk of HIV by 96%.
LEN now enters a select category, one of five ARV-based options for PrEP that all protect against HIV if you take them. But many of the people applauding the results from PURPOSE 1 and 2 will tell you that breakthrough science like this is, as hard as it is, is still the easy part. To break the back of the HIV epidemic demands overcoming an altogether different challenge—coordinating and accelerating every step in rolling out new products so that everyone who needs HIV prevention can get it.
Listen to this podcast to learn what must be done to finally deliver on the promise of highly effective HIV prevention, from pills to rings to injectable PrEP and beyond.
Listen
Resources
- Second Pivotal Trial of Twice-Yearly HIV Prevention Injection Safe and Highly Effective: PURPOSE 2 Trial Among Gay Men, Trans and Nonbinary People, AVAC Press Release
- The Lens on LEN: The basics on injectable lenacapavir as PrEP, AVAC
- Country planning matrix, PrEPWatch
- Moving a Product to the Real World, AVAC
- The long wait for long-acting HIV prevention and treatment formulations, Lancet
- A game-changer for PrEP if access is adequate, Lancet
- Allocation of Non-Commercial CAB for PrEP Supply in Low- and Middle-Income Countries, 2023-2025, AVAC
- Generic Cabotegravir Timelines, AVAC
- UNAIDS Exec Summary, UNAIDS
- Lenacapavir: What it would it take to get the 6-monthly anti-HIV jab to SA, Bhekisisa
Second Pivotal Trial of Twice-Yearly HIV Prevention Injection Safe and Highly Effective
AVAC welcomes the groundbreaking results of the PURPOSE 2 HIV prevention study of twice-yearly injectable lenacapavir for PrEP among 3,200 cisgender men, transgender men, transgender women, and nonbinary individuals who have sex with partners assigned male at birth. Among more than 2,000 people in the trial who received lenacapavir, there were only two HIV infections.
PxPulse: An Advocacy Chronicle on U=U in South Africa with Mandisa Dukashe

On this episode of PxPulse: The Advocacy Chronicles, Mandisa Tyadi Dukashe, Treatment Technical Lead at the South African National AIDS Council (SANAC) describes her work in helping to inspire and launch a National U=U Campaign in South Africa in 2024.
What is U=U? Undetectable equals Untransmittable refers to a major breakthrough in the science and understanding of HIV treatment. From data published in 2019, researchers confirmed that people living with HIV who have an undetectable viral load and take medication as prescribed have zero risk of transmitting HIV to their sexual partners.
This evidence galvanized a worldwide campaign, with the hope that spreading the word, Undetectable equals Untransmittable, would reduce stigma around HIV and inspire people to maintain their treatment.
The WHO released guidance in 2023 on how to monitor viral load, which provided supportive global policy for ministries of health. But WHO action is not enough to set public health policy or fulfill the promise of U=U.
Mandisa has long list of credentials behind her: She’s the co-founder of the U=U Africa Forum, part of the U=U Global Community Advisory Board, an alumnus of the AVAC Cure Research program and the AVAC Advocacy Fellows Program. Among many accolades, she has also been recognized as a 2021 Amazing People Living with HIV by HIV Plus Magazine.
Listen
Resources
Pandemic Accord Priority for 2025
As members of the Coalition of Advocates for Global Health and Pandemic Preparedness, a group of organizations advocating for an integrated and holistic approach to preparedness that emphasizes equity, inclusion, and synergies of multiple global health programs in advancing preparedness, we share the following asks with regard to the ongoing Pandemic Accord negotiations after their extension past May 2024.
Harnessing private sector strategies for family planning to deliver the Dual Prevention Pill, the first multipurpose prevention technology with pre-exposure prophylaxis, in an expanding HIV prevention landscape
Working with colleagues, AVACers Cat Verde Hashim and Kate Segal published this research article in the Journal of the International AIDS Society. The researchers undertook qualitative research in Kenya, South Africa and Zimbabwe to prioritize private sector service delivery approaches for the introduction of the DPP.
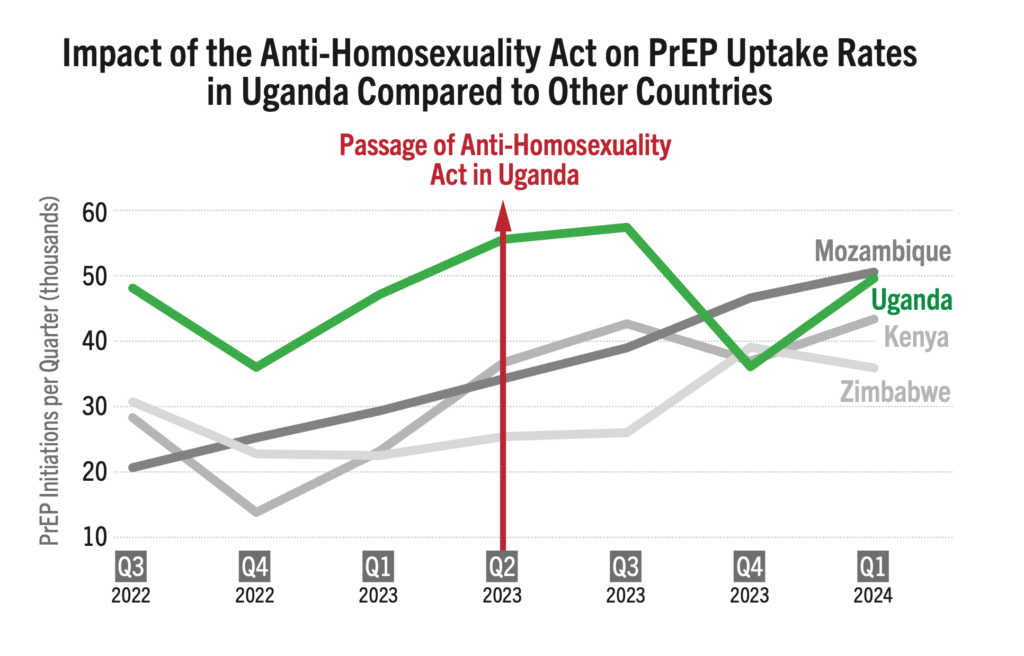
Impact of the Anti-Homosexuality Act on PrEP Uptake Rates in Uganda Compared to Other Countries
Oral PrEP initiations in Uganda were among the highest in the region, with sizable increases each quarter, until the enactment of the Anti-Homosexuality Act (AHA) in March 2023. Since then, the number of new PrEP initiations plummeted, and have since struggled to sustain rates seen in 2023. Public policies clearly matter.
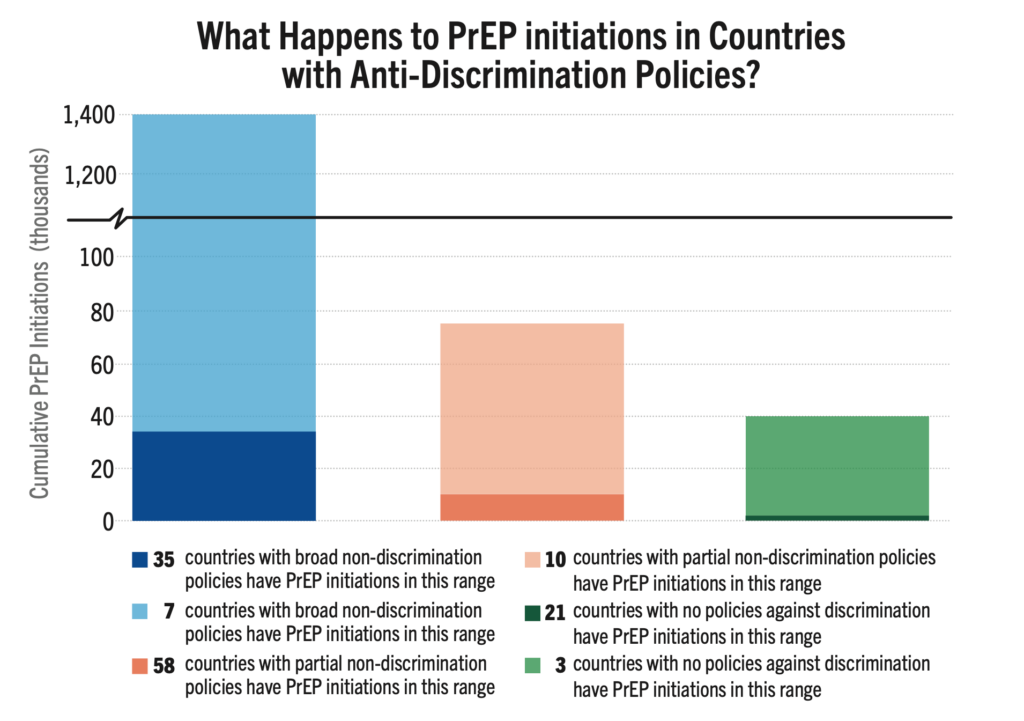
What Happens to PrEP Initiations in Countries with Anti-Discrimination Policies?
Among 134 countries reviewed, 42 have comprehensive anti-discrimination policies covering a broad range of populations, while 24 lack any such policies. The remaining countries have adopted partial measures. Nations with comprehensive anti-discrimination policies, document significantly higher rates of PrEP initiation compared to those without such protections. Key studies show a strong link between supportive policies (which can enable PrEP eligibility, HIV self-testing, and lower age of consent for treatment, for example) and higher PrEP initiations.
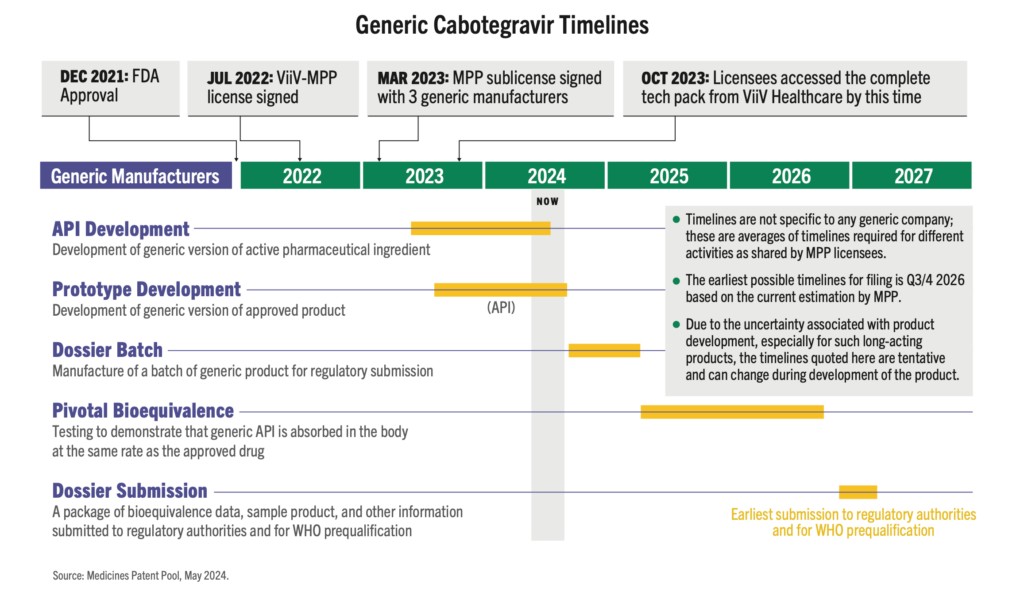
Generic Cabotegravir Timelines
As CAB for PrEP is a long-acting, extended-release injection, bioequivalence (BE) testing takes time to determine whether the generic drug functions in the body similarly to the original drug. As shown in this timeline graphic, the BE study (as determined by WHO guidance) is the longest part of the development process for generic CAB. But other steps, such as selecting and licensing generic manufacturers and technology transfer could be done faster.
PxWire Volume 14, Issue No. 3
PxWire is AVAC’s quarterly update covering the latest in the field of biomedical HIV prevention research and development, implementation and advocacy. Each issue includes updates, emerging issues and upcoming events. A PDF version of this report is also available.
Progress in PrEP Uptake
Oral PrEP initiations in Uganda were among the highest in the region, with sizable increases each quarter, until the enactment of the Anti-Homosexuality Act (AHA) in March 2023. Since then, the number of new PrEP initiations plummeted, and have since struggled to sustain rates seen in 2023. Public policies clearly matter.
Among 134 countries reviewed, 42 have comprehensive anti-discrimination policies covering a broad range of populations, while 24 lack any such policies. The remaining countries have adopted partial measures. Nations with comprehensive anti-discrimination policies, document significantly higher rates of PrEP initiation compared to those without such protections. Key studies show a strong link between supportive policies (which can enable PrEP eligibility, HIV self-testing, and lower age of consent for treatment, for example) and higher PrEP initiations.
An analysis presented at AIDS 2024, HIV Pre-Exposure Prophylaxis Policies Worldwide, by the Georgetown University Center for Global Health Policy & Politics and AVAC found that 69% of the 194 countries reviewed have approved at least one PrEP product. However, only 52% of these countries adhere to WHO guidance on PrEP eligibility, with regulatory approvals and national policies that support PrEP use for all populations or people at risk. This data reinforces the critical need for policies that support PrEP uptake.
PrEParing for New Products
Advocacy to accelerate licensing for new products can speed the process of generics manufacturing.
Three generic manufacturers—Aurobindo, Cipla, and Viatris—have licenses via the Medicinces Patent Pool to develop generic versions of injectable cabotegravir (CAB) for PrEP. These generic products are currently in development and expected to be submitted to regulatory authorities in the second half of 2026, with possible approval in 2027.
Does the Timeline Have to be this Long?
As CAB for PrEP is a long-acting, extended-release injection, bioequivalence (BE) testing takes time to determine whether the generic drug functions in the body similarly to the original drug. As shown on the timeline graphic below, the BE study (as determined by WHO guidance) is the longest part of the development process for generic CAB. But other steps, such as selecting and licensing generic manufacturers and technology transfer could be done faster. It is critical that generic manufacturers for new PrEP products, such as lenacapavir, star as soon as possible. This requires Gilead to pursue licensing even before regulatory submission, and to accelerate technology transfer and API development.
Product Updates
- Data from HPTN 084 show that CAB for PrEP was generally well tolerated and safe for both pregnant cisgender women and their babies.
- China has approved CAB for PrEP.
- With PEPFAR support, Eswatini, Nigeria, and Ukraine are poised to introduce CAB for PrEP into their national PrEP programmes this quarter, and the South African government announced CAB supplies from PEPFAR will be launched in Q1/Q2 2025.
See AVAC’s Planning Matrix for more!
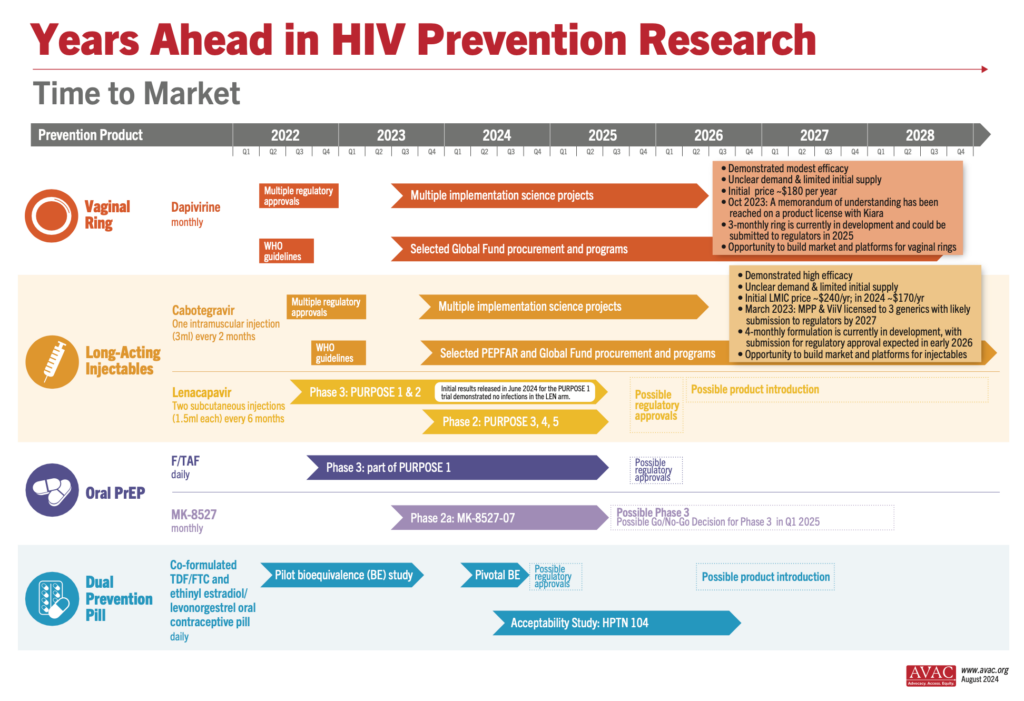
The Latest R&D in the Prevention Pipeline
Results from the PURPOSE 1 trial of injectable lenacapavir showing 100% efficacy in preventing HIV among cisgender women and adolescent girls dominated headlines at the AIDS 2024 conference. This Phase 3 trial, conducted by Gilead Sciences, involved over 5,000 participants from South Africa and Uganda and demonstrated superior results compared to daily oral PrEP options. See the New England Journal of Medicine publication, accompanying Editorial and our advocates’ primer to learn more about the results and what needs to happen next: Lens on LEN: The basics on injectable lenacapavir as PrEP.
Gilead’s PURPOSE 2 Phase 3 trial is ongoing and testing injectable lenacapavir as PrEP in the US, South Africa, Peru, Brazil, Mexico, Argentina, and Thailand, among cisgender men who have sex with men, transgender women, transgender men, and gender non-binary individuals. With 3,000 participants enrolled, initial results are anticipated in late 2024 or early 2025. Two other trials (PURPOSE 3 & 4) are currently recruiting in the United States, enrolling cisgender women and people who inject drugs, respectively. Enrollment for PURPOSE 5 in France and the UK is expected to commence in the latter half of this year. For more information about the lenacapavir trials, visit: An Overview of Lenacapavir for PrEP Trials.
Meanwhile, the bioequivalence study for the Dual Prevention Pill (DPP), designed to prevent both HIV and pregnancy, has concluded successfully, with each of the drugs functioning together in the body in a similar way to how they function alone. The promising results pave the way for regulatory submission and potential large-scale adoption, offering another significant tool in the fight against HIV and unintended pregnancies. Learn more about the DPP and find resources for your advocacy.
Prevention Playlist
AVAC develops a wide range of resources to inform decision making and action. Check out the latest:
Join
- 2024 STI Prevention Conference
- 2024 IUSTI World Congress
- HIV Research for Prevention (HIVR4P) Conference 2024
Read
- Choice, Access and Equity at AIDS 2024
- LEN in the Spotlight at AIDS 2024
- First Full Day of AIDS 2024
- AIDS 2024 Preconference Highlights
- Frontiers in Reproductive Health Special Issue: Multipurpose Prevention Technologies
- Global HIV Prevention Advocates Call for Accelerated Timeline for Widespread Access to Injectable Lenacapavir for PrEP
- Landmark Trial in South Africa and Uganda Finds Twice Yearly HIV Prevention Injection Safe and Highly Effective
- The Pandemic Accord: A critical fight in 2024
- Pride and a Transnational Anti-LGBTQ+ Reaction
- Tracking PrEP Rollout & Learning Lessons
- Pre-Exposure Prophylaxis and Adolescent Girls and Young Women in Eastern and Southern Africa: The latest insights
Watch and Listen
- Where’s the Equity? Including People with Disabilities in HIV Prevention Programming and Research
- PxPulse: The Advocacy Chronicles with Ruth Akulu who shares advocacy wins around the Dual Prevention Pill
- PxPulse: The Advocacy Chronicles with SMUG’s Allan Mwasa who discusses Uganda’s anti- homosexuality legislation
- You Get What You Measure: Why Monitoring for PrEP Choice Helps Tell Our Story
- PrEP Justice: Updates on the US v. Gilead case and the fight for equitable PrEP access
- AIDS 2024: New Ways for the Next Wave: Innovative R&D for the future of Women’s prevention
- Responding to Project 2025’s Threats to Science, Rights and Resources
- Illinois PrEP Summit 2024: Disrupting Disparities and Advancing Access
- It’s Not Just about the Trial: GPP from discovery to delivery in TB research
- From the Lab to the Jab: Lessons learned and what’s next in HIV vaccine research
- What’s Next for the Pandemic Accord? A civil society and communities perspective
- PrEP and the Role of HIV Self-Testing
Use
- Advocates’ Guide to Multipurpose Prevention Technologies (MPTs)
- Lens on LEN: The basics on injectable lenacapavir as PrEP
- The GPP Body of Evidence: GPP Monitoring and Evaluation Frameworks, REAL and REAL2
- Advocates’ Guide to DoxyPEP
- Using the COMPASS Campaign Advocacy Assessment Tool (C-CAAT) to assess the effectiveness of advocacy campaigns
- Injectable Cabotegravir Evidence Gap Tracker
