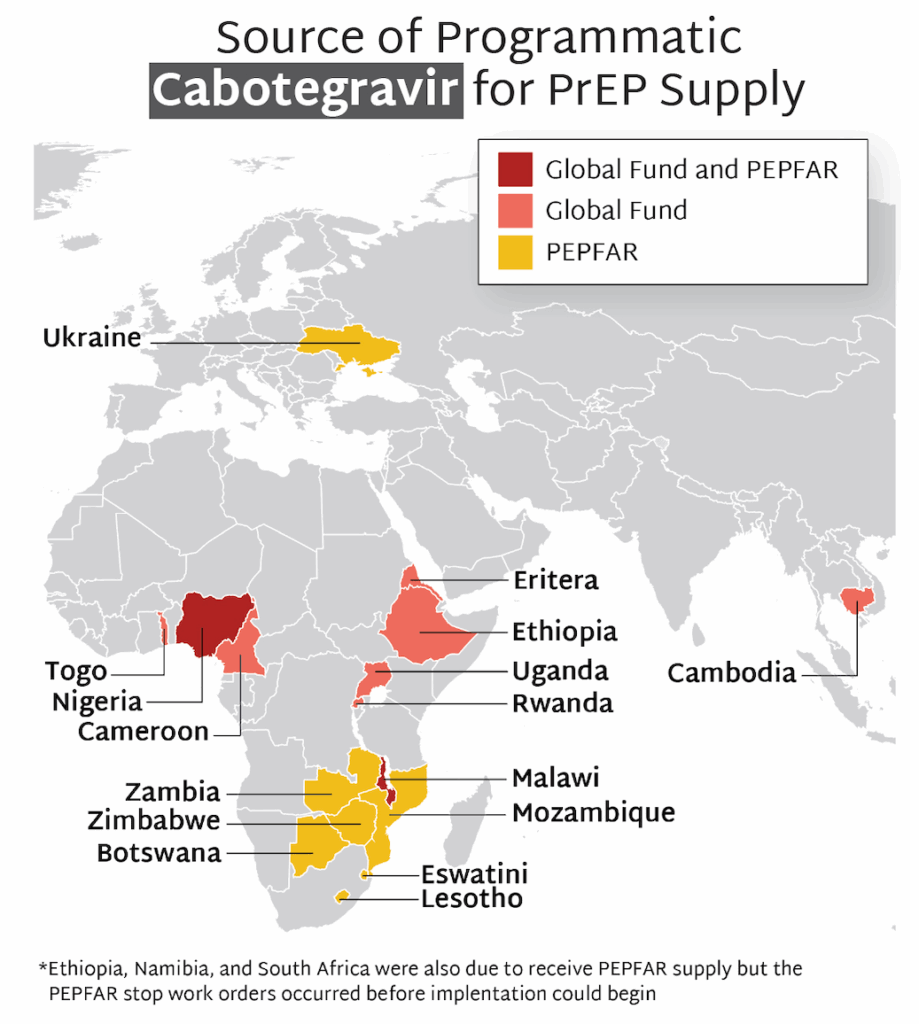
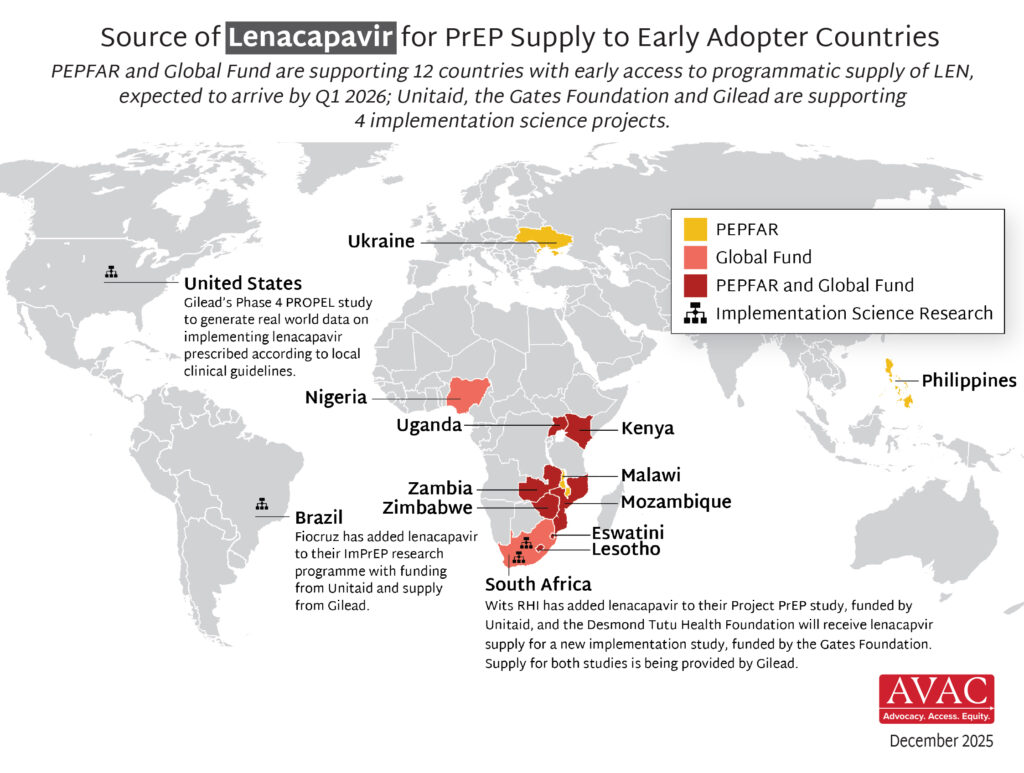
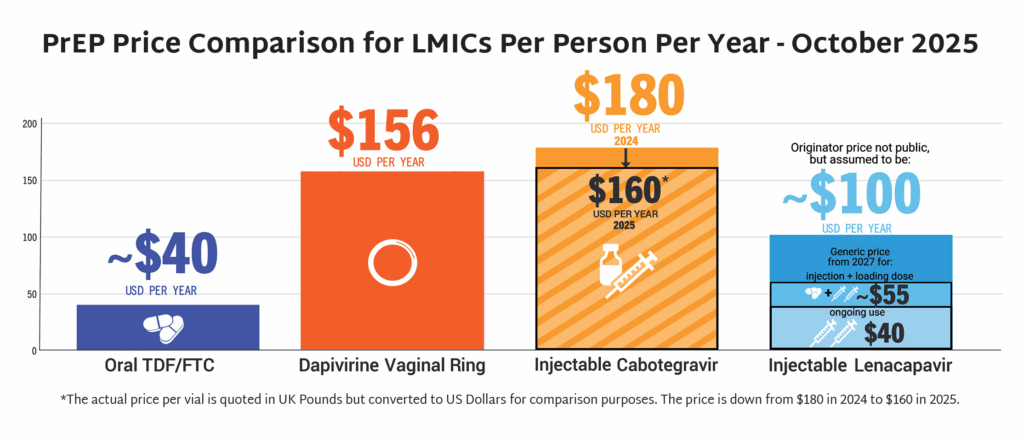
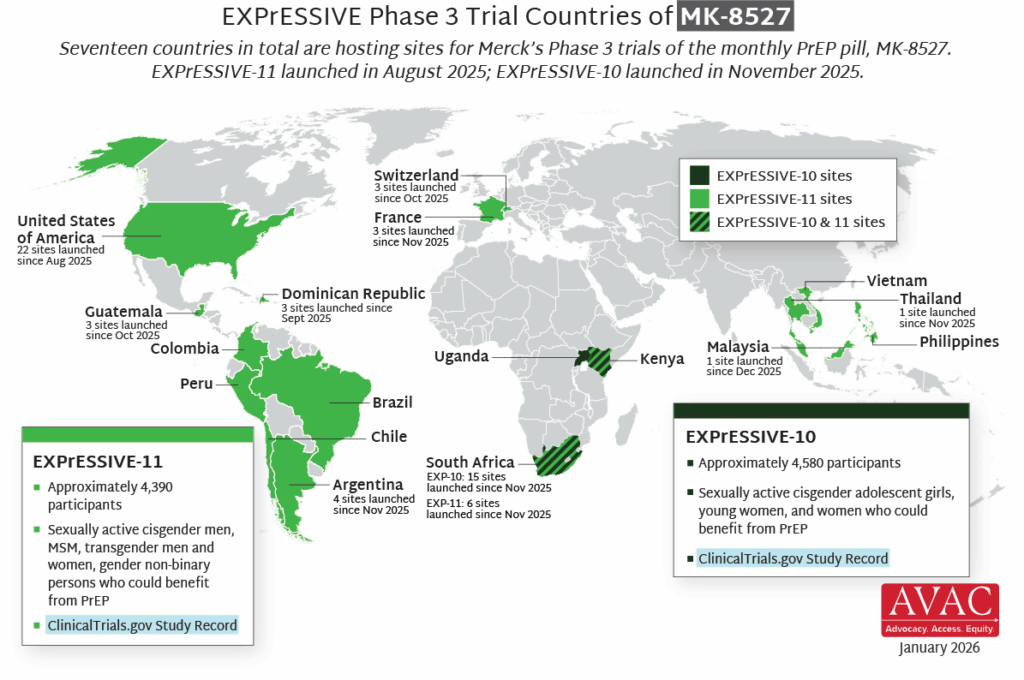
In the first year since efficacy results were reported, LEN for PrEP has achieved critical milestones in record time compared to oral PrEP, the dapivirine vaginal ring, and injectable cabotegravir. Speed, scale, and equity are still needed to deliver impact.
View the interactive version on our comprehensive page on all things lenacapavir. And the animated GIF version is available for download.