Programs for delivering PrEP have been shuttered all over the world by the withdrawal of the US government from global health. This graphic illustrates some of the severe measurable impacts of these cuts. Excerpted from PxWire.
PrEP Delivery Imperiled
PxWire Volume 15, Issue 2
The field of HIV prevention is confronted with two opposing forces; programs for delivering PrEP have been shuttered all over the world by the withdrawal of the US government from global health. At this same moment in history, next-generation long-acting products hold great promise to accelerate HIV prevention and help the world achieve epidemic control. Navigating these seismic developments requires unprecedented coordination, solidarity, and courage.
Global health champions can defy the hatred, fear, and greed that are dominating politics in so many places around the world. Together we can innovate, create, and protect the advance of HIV prevention and global health. This issue provides a snapshot on threats to delivering PrEP, the potential of injectable lenacapavir (LEN) for PrEP, and on the implications of upstream research and development of other long-acting PrEP.
Read below or download the PDF version.
Progress in PrEP Uptake: Threatened
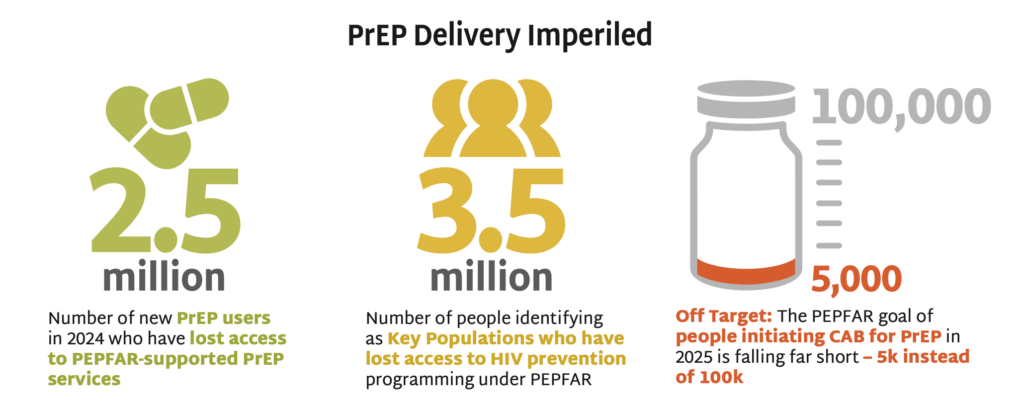
- PEPFAR documented 2.5 million new PrEP users in 2024, who could now lose access to PEPFAR- supported PrEP services. US Department of State issued a limited and inconsistently implemented waiver in February, allowing for continued provision of HIV treatment but restricting PrEP access to pregnant and lactating people only.
- These actions will result in 3.5 million who identify as key populations (KPs) losing access to all HIV prevention programming under PEPFAR, according to 2024 PEPFAR data tracking KP use of PrEP. These groups have higher rates of HIV incidence and face additional barriers to accessing services now that targeted programs are suspended.
- PEPFAR had a goal of 100,000 people initiating cabotegravir (CAB) for PrEP in 2025. But only 5,000 individuals had initiated by October 2024. The suspension of PEPFAR funding imperils scale-up of this long-acting product.
- These figures represent just some of the disruptions that are decimating PrEP delivery. Learn more here: Impact of PEPFAR Stop Work Orders on PrEP
- Overcoming this challenge, restoring, sustaining, and accelerating PrEP access is imperative and possible if the field works together.

For the last eight years, AVAC has proudly worked with PEPFAR to document PrEP uptake and its impact around the world. That stopped in January with a stop work order from the US government. But protecting access to PrEP is vital. Are you leading a PrEP program? Whether supported by PEPFAR or not, we invite you to work with us to ensure global data on PrEP is not lost. Find us at [email protected].
PrEParing for New Products
- Approval by the US Food and Drug Administration (FDA) for injectable 6-month LEN for PrEP is expected in June, with WHO guidelines expected in July. See the full timeline.
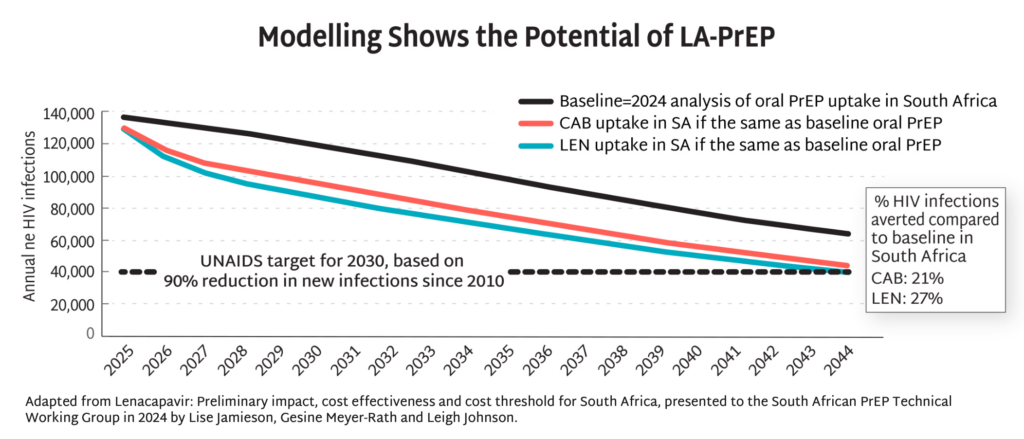
- Modelling data from South Africa demonstrate the potential of injectable PrEP to dramatically reduce HIV incidence by up to 90% by 2044, and potentially even sooner with more aggressive uptake. This potential goes beyond South Africa, lighting the way toward epidemic control the world over.
- The field must be prepared for swift action once LEN is approved and recommended, to ensure this opportunity is not squandered. As AVAC’s interactive timeline, Tracking LEN Rollout, outlines, donors, ministries of health, manufacturers, regulators, and civil society all have a role to play to pave the way for swift, equitable and effective introduction of LEN for PrEP.
The Latest R&D in the Prevention Pipeline
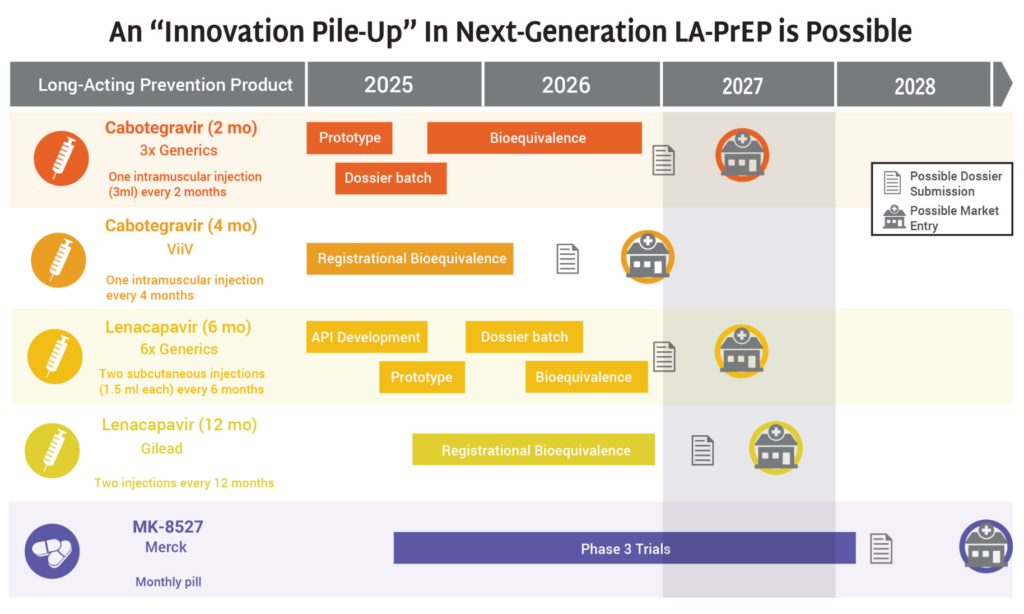
- The HIV prevention market is headed toward a period of significant opportunity—and possible congestion—as a slate of new products are on track for continued development and potential introduction to the market in 2027 and 2028.
- Generics for 2-month CAB and 6-month LEN, along with ViiV’s 4-month CAB, Gilead’s 12-month LEN, and Merck’s monthly oral MK-8527 PrEP pill (if further development and approvals are successful) could all enter the market by 2028.
- The possibility of so many products on the market, including four different formulations of injectable PrEP, means that it is imperative the field prepares for this future now.
- Markets and policies must be built to support the products in the market already, so that new options can be rapidly deployed and deliver impact. Otherwise, the field will squander time and money, with epidemic control slipping further out of reach.
- With US funding cuts to both HIV prevention R&D and delivery, communities must be engaged, supported, and informed about all prevention options, and the choices that all stakeholders will need to make. This means gathering and sharing data and information about cost-effectiveness, user acceptability, program feasibility, and impact. Communities empowered with the facts can advocate for the choices they need, and push ministries of health to make strategic investments and procure the prevention method mix that fits their context and delivers impact.
Prevention Playlist
AVAC develops a wide range of resources to inform decision making and action. Check out the latest:
Join
- Subscribe to Global Health Watch: AVAC’s weekly newsletter dedicated to breaking down critical developments in US policies and their impact on global health, at avac.org/global-health-watch
- CHANGE: In response to the unfolding crisis, more than 1,500 people from civil society organizations around the world have launched CHANGE—Community Health & HIV Advocate Navigating Global Emergencies—a coalition formed to support urgent action: [email protected]
Use
- Research Matters Advocacy Toolkit, AVAC
- Tracking the Freeze: Real-Time Impact on Key Populations, GBGMC
- Impact of the Stop-Work Order on PrEP, AVAC
- Tracking Lenacapavir Rollout, AVAC
- PEPFAR Program Impact Tracker, Impact Counter
- Weekly Situation Report, UNAIDS
Watch/Listen
- Politics and Global Health: The Need for a New, Resilient Architecture, Webinar
- Lawsuit Wins and What’s at Stake: AVAC v US Department of State, PxPulse episode
- Global Health in the Lurch: What’s happening now and who is pushing back, PxPulse episode
- Advancing Sexual and Reproductive Health and Rights: Moving forward post-the 2024 US Election, Webinar
Read
- Despite USG Global Health Collapse, Here Are Several Data Trackers To Support Your Advocacy, AVAC
- On Going Backwards, Lancet
- The Trump Administration’s Foreign Aid Review: Status of PEPFAR, KFF
- The Best Investment You Didn’t Know You Made: How NIH Funding Fuels Innovation and Economic Growth, amfAR
- HIV Market Impact Memo, CHAI
- The USAID List of Terminated Global Health Awards – What Does it Tell Us?, KFF
- AVAC Condemns HHS Mass Layoffs
- PrEP in the Balance: Hopes and fears in 2025
- Better Engagement, Better Evidence: Working in partnership with patients, the public, and communities in clinical trials with involvement in good participatory practice, Lancet
- PEPFAR: A Strategic Necessity for US Leadership and Global Health, AVAC
- Rallying for HIV Prevention Amid Policy Attacks, AVAC
- CROI 2025 Shows the Promise of Research at its Best, AVAC
AVAC and FAPP Written Statement: US Senate Hearing on Biomedical Research
AVAC and FAPP submitted written testimony for the US Senate Appropriations Subcommittee’s April 30th hearing, “Biomedical Research: Keeping America’s Edge in Innovation.”
A set of suggested questions for the Senators to consider was also included in hopes of spotlighting key issues around the importance of lifesaving research conducted by the NIH—specifically in the area of HIV—and its critical role in health equity and innovation. We hope that this can be entered into the record for this hearing.
Research Matters Advocacy Toolkit
This toolkit for researchers shares key messages, practical advocacy guides, and resources to help move our collective efforts forward.
Lawsuit Wins and What’s at Stake
On February 10, AVAC led other organizations to sue the US government including the President, the US State Department and USAID, seeking emergency relief from an Executive Order that inhumanely froze all funding for foreign assistance. This case may well help to determine the future of foreign assistance, executive overreach, and the role of evidence, facts, and values in US policy.
AVAC’s Executive Director, Mitchell Warren and Public Citizen Litigator, Lauren Bateman explain these lawsuits and why they matter.
Resources
- AVAC v. United States Department of State, AVAC
- AVAC press releases summarizing key decisions in the case, AVAC
- Global Health Watch, AVAC
- All public documents related to the case, Court Listener
- Impact of US Funding Cuts, UNAIDS
- Viewpoint: Testing the Boundaries of Presidential Powers in Health, JAMA
The Role of PEPFAR in HIV Prevention
AVAC Senior Program Manager for Policy, John Meade Jr., describes PEPFAR’s historic legacy and strongly argues for its continued importance in the face of attacks by the new US administration. This piece appears in The Broadsheet, the magazine published by the Congressional Black Caucus Health Braintrust.
Better Engagement, Better Evidence
Writing in the Lancet Global Health, AVAC staffers Stacey Hannah, Jessica Salzwedel, and several co-authors, write about the importance of community stakeholder engagement clearly seen after World Health Organization adoption of new rules requiring clinical trials to improve this kind of coordination.
Self-Care Advocacy for HIV and STI Prevention
Self-care, the ability of people to promote and maintain health, prevent disease, and cope with illness and disability with or without the support of a healthcare worker is especially critical now, as the new US administration’s sweeping funding cuts and policy shifts threaten to erode support for traditional healthcare services, including HIV and STI programs.
By putting testing, prevention, and treatment directly into people’s hands, self-care can help communities maintain vital health services despite reduced funding, limited access to healthcare, and diminished government support. Read our new guide, Self-Care Advocacy for HIV and STI Prevention, on STIwatch.org.
Global Health in the Lurch: What’s happening now and who is pushing back?
KFF’s Jen Kates and AVAC’s John Meade break it all down on PxPulse Live.
A snapshot of global Health in the first weeks of the Trump Administration, this episode covers the impact of the US freeze on foreign aid to critical federal agencies and the HIV research pipeline and explores action in Congress and among civil society to push back.
Resources
Trials & Projects Halted by USAID Funding Suspension
The stop-work orders have disrupted USAID-supported HIV prevention research, halting critical investigations in vaccine and next-generation PrEP strategies.
The abrupt suspension of these trials also raises serious ethical concerns. Stopping trials mid-course undermines trust in research, jeopardizes community engagement, and abandons participants who volunteer their bodies for scientific discovery. It will take years to build back this critical infrastructure—for HIV research and beyond—as well as the community partnership and trust needed to ensure smooth and ethical research.