
When AVAC was founded in 1995, we were called the AIDS Vaccine Advocacy Coalition. Our singular goal was to advance swift, ethical research for a vaccine that was then — and is today — essential to bring the epidemic to a conclusive end.
Twenty years later, AVAC is still focused on swift and ethical research, but our scope has expanded. Along with vaccines, we advocate for PrEP, microbicides, voluntary medical male circumcision and more.
Through it all, our message has been the same: prevention is the center of the AIDS response. Not just any prevention but smart, evidence-based, community-owned, rights-based strategies.
We do this work because it’s essential. We are able to do it because of our robust partnerships worldwide. We will keep doing it — with your help — until the epidemic has, finally, come to an end.

 We’ve experienced 20 years of breakthroughs and disappointments in prevention research. A vaccine that many had given up on was the first to provide modest protection. One microbicide everyone hoped for didn’t pan out. Male circumcision and PrEP studies overcame skepticism and, together with antiretroviral therapy, paved the way for a prevention revolution.
We’ve experienced 20 years of breakthroughs and disappointments in prevention research. A vaccine that many had given up on was the first to provide modest protection. One microbicide everyone hoped for didn’t pan out. Male circumcision and PrEP studies overcame skepticism and, together with antiretroviral therapy, paved the way for a prevention revolution.
Through it all, AVAC has worked with partners to maintain the field’s focus and press for continued research into an AIDS vaccine, a cure and more.

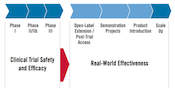 When AVAC was founded, the only biomedical HIV prevention options for adults were male and female condoms. The pathway for introducing any new strategy was largely unmapped. No one knew where the gaps would be—between trial result and country action, between guidance and financial support. Now we do.
When AVAC was founded, the only biomedical HIV prevention options for adults were male and female condoms. The pathway for introducing any new strategy was largely unmapped. No one knew where the gaps would be—between trial result and country action, between guidance and financial support. Now we do.
Over two decades, AVAC has not only identified the gaps; we’ve worked to bridge them, so that products reach people in programs that work — without delay.

 Twenty years ago, advocacy for HIV prevention hardly existed. So AVAC helped build a global network of advocates equipped with effective advocacy strategies and the latest evidence.
Twenty years ago, advocacy for HIV prevention hardly existed. So AVAC helped build a global network of advocates equipped with effective advocacy strategies and the latest evidence.
With our support, they are putting prevention on the agenda in countries and communities around the globe.

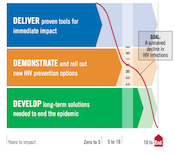 When the world lacked a plan for ending AIDS, we helped create one.
When the world lacked a plan for ending AIDS, we helped create one.
Now we’re holding global leaders accountable for results — demanding the resources, policies and evidence-based plans needed to deliver all of today’s prevention options to the people who need them, and to plan for the rapid rollout of new options as they emerge.

 Communities’ support for prevention research can never be taken for granted — it has to be earned. For 20 years, we’ve helped build trust between researchers, funders and communities to speed the ethical development and rollout of new prevention options.
Communities’ support for prevention research can never be taken for granted — it has to be earned. For 20 years, we’ve helped build trust between researchers, funders and communities to speed the ethical development and rollout of new prevention options.
And when controversy threatened to derail those efforts, AVAC provided leadership and resources to help get them back on track.

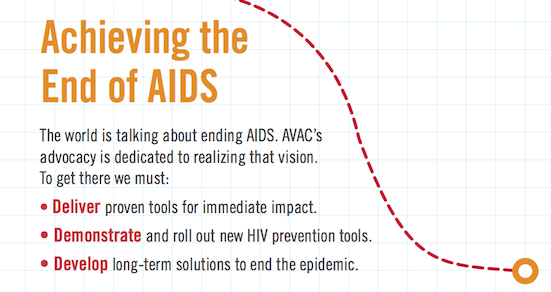
Your gift to AVAC will support our efforts to accelerate the development and delivery of HIV prevention options to men and women worldwide. With your help, we can continue to convene, collaborate and communicate a strong, clear and cohesive vision for HIV prevention today, tomorrow and to end the epidemic.
It will take all of us working together to end AIDS. Please join us.


