DREAM-01
MTN 003D
Population Council #558
Cabotegravir implant (Preclinical)
Access to the Dapivirine Vaginal Ring: A timeline on progress
New Issue of PxWire!
PxWire is AVAC’s quarterly update covering the latest in the field of biomedical HIV prevention research and development, implementation and advocacy. Each issue includes news, emerging issues and features upcoming events.
The HIV field gathers for its first hybrid International AIDS Conference (IAC) since the start of COVID-19 pandemic at a pivotal moment in HIV prevention. Across research to rollout – accelerated product access, new products reaching the market, new trials starting (and pausing) and recent research results – the ability to deliver two new proven PrEP methods will be determined by conversations and decisions happening now.
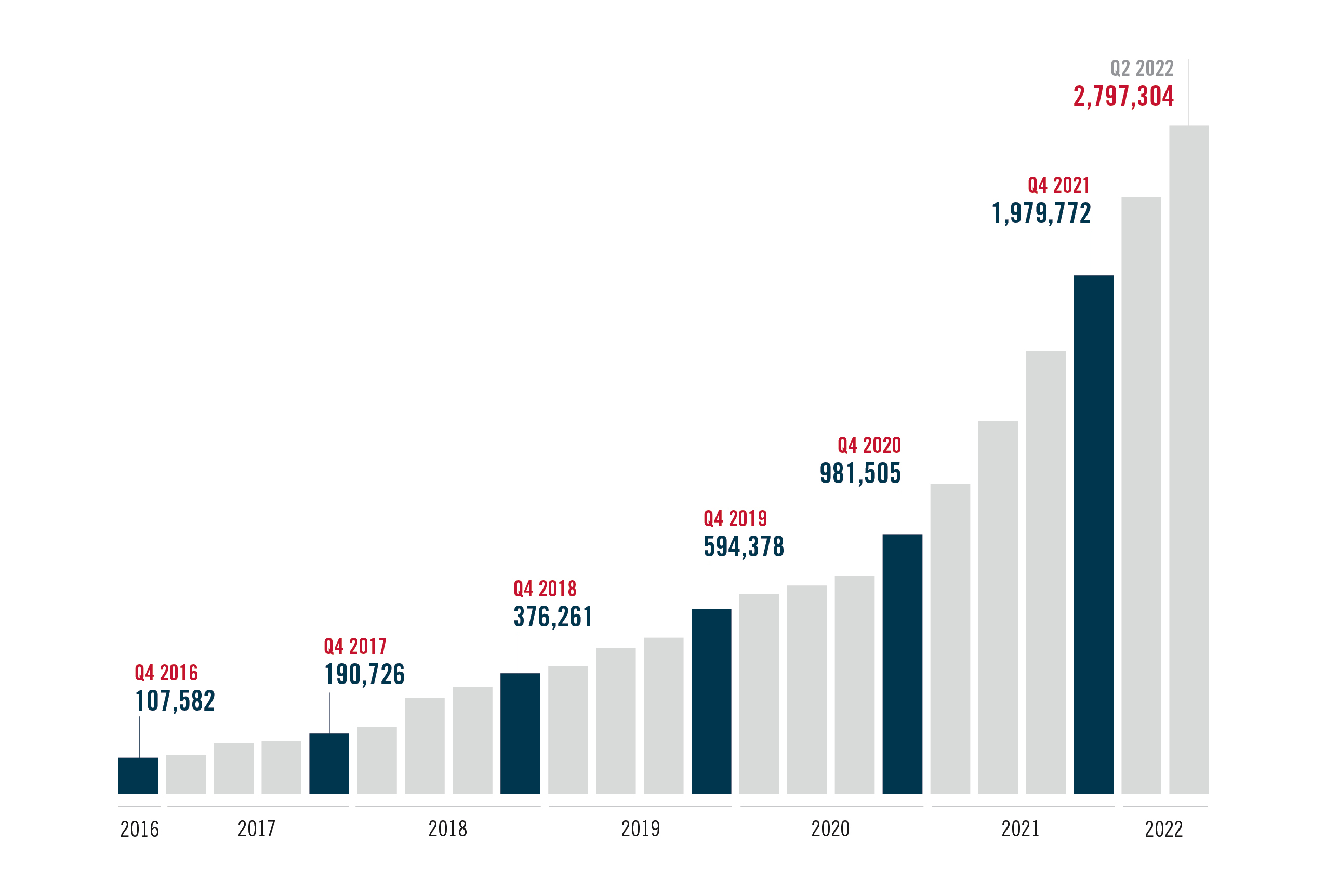
After initial approval ten years ago, oral PrEP initiations have surpassed 2M globally, reaching 2,797,304 – with significant progress over the past year, but still well below UN targets.
Check out the full issue of PxWire here and scroll down for important updates.
Welcoming Amb. John Nkengasong to PEPFAR, and 3 Priorities
Yesterday, Dr. John Nkengasong was sworn-in as the US Global AIDS Coordinator and Special Representative for Global Health Diplomacy overseeing PEPFAR, the largest funder of HIV/AIDS programming in the world. We are confident that with Ambassador Nkengasong’s ambitious leadership, vision and experience, PEPFAR and the global community can maintain the urgency and impact in ending the AIDS pandemic, continue responding to COVID, and build the sustainable health infrastructure that we so desperately need.
We also want to thank Angeli Achrekar for her steadfast commitment to end the AIDS pandemic and her terrific leadership over the past two years.
At AVAC, our eyes are on three major actions to help us get there these next few months – and urge Ambassador Nkengasong’s and PEPFAR to take immediate and bold actions to:
- Leverage new biomedical prevention options to re-boot primary prevention – and make sure these new options become viable choices for all people who can benefit from them. Accelerating access and introduction of injectable CAB for PrEP and ensuring political and financial support to also introduce the dapivirine vaginal ring provide an opportunity to build comprehensive, sustainable and integrated prevention programmatic platforms to deliver choice today and build for a future of even more options.
- Center communities in the prevention response. Generating deeper, more consistent community engagement in prevention requires restructuring community engagement. Community input must move beyond “consultation” on specific questions or challenges to a deeper, more meaningful, sustained, strategic engagement that supports the full navigation of challenges from research to rollout, in all their complexity.
- Imbed the AIDS response into the future of global health security and pandemic preparedness and response (PPR). The certainty of more complex and challenging health crises on the horizon demands rights-based public health approaches that focus on health systems strengthening, comprehensive integration of key health priorities including HIV, TB, malaria, sexual and reproductive health and non-communicable diseases and lay effective groundwork for addressing emerging health priorities in the future. And efforts to build a global pandemic response capacity must leverage the organizational capacities, investments, and reach of PEPFAR and the Global Fund to Fight AIDS, TB and Malaria.
There remain enormous challenges and opportunities ahead. We are very excited to welcome Ambassador Nkengasong back to PEPFAR and for his leadership in the global AIDS response. We look very forward to working with him toward our shared goals of ending the AIDS epidemic and ensuring global health equity.
Statement on the Dapivirine Ring for Women: Call for Accelerated Global Access
This statement, from a coalition of advocates, applauds the WHO for its ongoing support and its 2021 recommendation of the dapivirine vaginal ring as an additional prevention option for women. The advocates call on funders, country governments and community leaders to sustain their support for the ring’s introduction and rollout in African countries where it is needed and for prompt regulatory reviews. And they call on HIV programs to integrate the ring, and collaborate with communities on the design of those programs.
Choice is key. Support the ring!
Choice is key. This is the theme this week – and always – in HIV prevention.
There is growing advocacy this week calling for greater political and financial support to introduce and roll out the dapivirine vaginal ring. HIV advocates from AEDC, APHA, DARE, Emthojeni, GNP+, ICWEA, PZAT, TALC, WACI Health, among others, are sounding the alarm that adding this option to existing strategies for HIV prevention will save lives and offer real choices to the people who need them most. The ring, now approved in a growing number of countries, offers women a discreet method of HIV prevention and one more option that may fit their needs best at any given time.

Chilufya Hampongo of TALC, a leading advocate for HIV prevention in Zambia, said during a WHO consultation earlier this week, “The ring empowers me. I want the chance to be offered the ring, and to find it when I look for it. Pills are not my thing. Injections are not my thing. I want the ring.”
The World Health Organization shared findings Tuesday from consultations with national ministries of health (MOHs), civil society, ring users, donors, implementing partners, and other key stakeholders in countries across Africa, which explored questions and priorities related to the introduction of the ring. The WHO reported on resounding support for the ring, a clear commitment to expanding the choices for HIV prevention options. These findings come amidst growing concern that leading funders will not support programs to procure and offer the ring.
During the WHO meeting – a summary is here – feminists and African advocates called on USAID, PEPFAR, the Global Fund and national governments to fund the ring.

Lillian Mworeko of ICWEA said, “40 years of crying and we don’t have the options we need for women facing HIV risk. How do we make the ring available to work toward ending AIDS by 2030?”
In a statement that followed the WHO consultation, a coalition of advocates applauded the WHO for its ongoing support and its 2021 recommendation of the ring as an additional prevention option for women. They call on funders, country governments and community leaders to sustain their support for the ring’s introduction and rollout in African countries where it is needed and for prompt regulatory reviews. And they call on HIV programs to integrate the ring, and collaborate with communities on the design of those programs.
In the coming days, weeks and months, funders and policy makers must hear this call. Advocates called on PEPFAR and USAID for a discussion and are also engaging decision-makers in their communities and countries.
And for more conversations like this one, check out the Choice Agenda, a new global forum devoted to advancing choice in HIV Prevention. Join the listserv, participate in the webinars, identify the priorities and help turn options into choices.
New Resources on the Science from CROI, the Ring, DPP and More!
In this week’s roundup, you’ll find details on an upcoming webinar covering the stand-out science from CROI 2022; an important read on vaccine equity; new resources to support advocacy for the dapivirine vaginal ring; resources for learning about and advocating for the Dual Prevention Pill; and a special tribute to Zena Stein.
Webinar: The most notable science at CROI
Wednesday, April 27, 2022 at 9:30am ET/15:00 SAST
Join us, the CROI Community Liaison Subcommittee, and European AIDS Treatment Group for a webinar to debrief on this year’s CROI. A fabulous panel will unpack findings from the conference, share perspectives, and offer advocacy suggestions. Topics will span science presented on HIV prevention and treatment, HIV and aging, HIV cure, and COVID-19. Register here. Recorded sessions from CROI 2022 are now available on demand. For context on the sessions, the recordings from the Margarita Breakfast Clubs explore findings from the research of the day at CROI.
Read Vaccine equity: The rollout that needs a booster shot
An opinion piece in The Hill newspaper by AVAC Executive Director Mitchell Warren points out connections in global health that cut across disease and must be addressed for biomedical products to reach those who need them most. “COVID-19 has shown us the fragility of our efforts to end diseases in places where poverty is entrenched…. We invest hundreds of millions in large trials but nothing similar on how to disseminate the results…. This is not unique to any one disease: We have seen it in the lack of global vaccine equity in the COVID-19 response and we’ve seen it in TB and HIV…. We are achieving breakthroughs in developing new medicines and vaccines, but we are failing to deliver them with equity and with impact. It is well past time to do better.”
Important Voices: Ring user speak out
A video series, developed by the USAID-funded MOSAIC Project, features the voices of women from trials in Kenya, South Africa, Zambia and Zimbabwe that tested the dapivirine vaginal ring. Trial participants explain why the ring works for them and their need for future access to this HIV prevention method.
Watch This Space: HIV and unintended pregnancy prevention
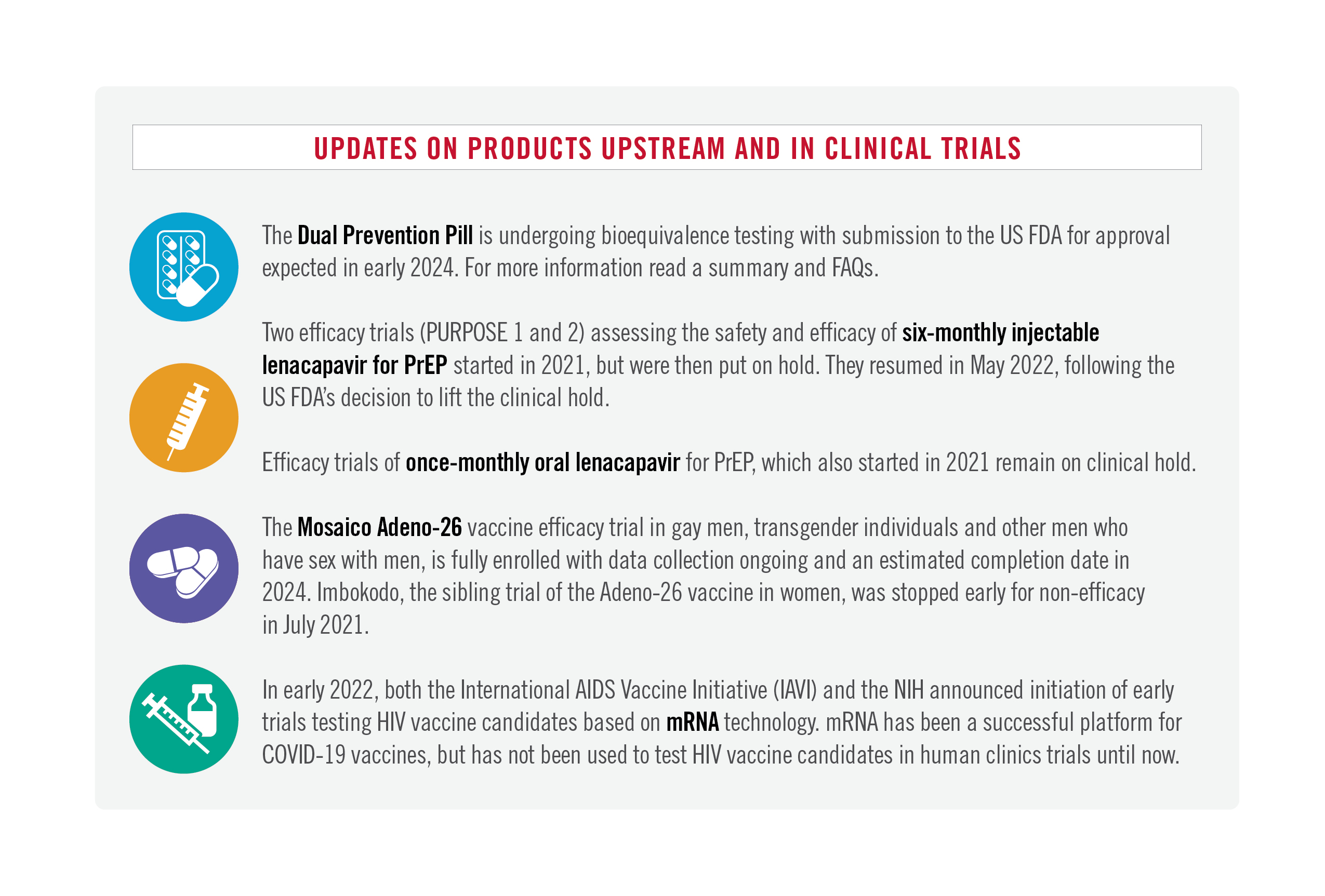
Last week, AVAC and FP2030 held a consultation on the Dual Prevention Pill (DPP), a daily pill that is currently being developed for the simultaneous prevention of unintended pregnancy and HIV acquisition. The webinar brought in dynamic perspectives from the family planning and sexual & reproductive health communities. Be sure to keep this on your radar, as there will be more opportunities at AIDS2022 to learn more, get your questions answered and develop advocacy priorities for this promising multipurpose technology. Currently, the DPP is moving through the R&D process and could be available as early as 2024. It would be the first MPT available. Get more details on the DPP on PrEPWatch.org.
Listen: Life and legacy of Zena Stein
To reflect on the legacy of Zena Stein, a public health, human rights and HIV prevention champion who influenced so many working in HIV prevention, the editor of the American Journal of Public Health produced a video-podcast conversation with CAPRISA’s Quarraisha Abdool Karim and AVAC’s Mitchell Warren.