At AVAC, we are seeing remarkable new developments and opportunities to engage in HIV and COVID research, development and advocacy. Here is a round-up of what’s been happening, essential reading and resources to help in your advocacy efforts, and some upcoming webinars to join.
In PrEP
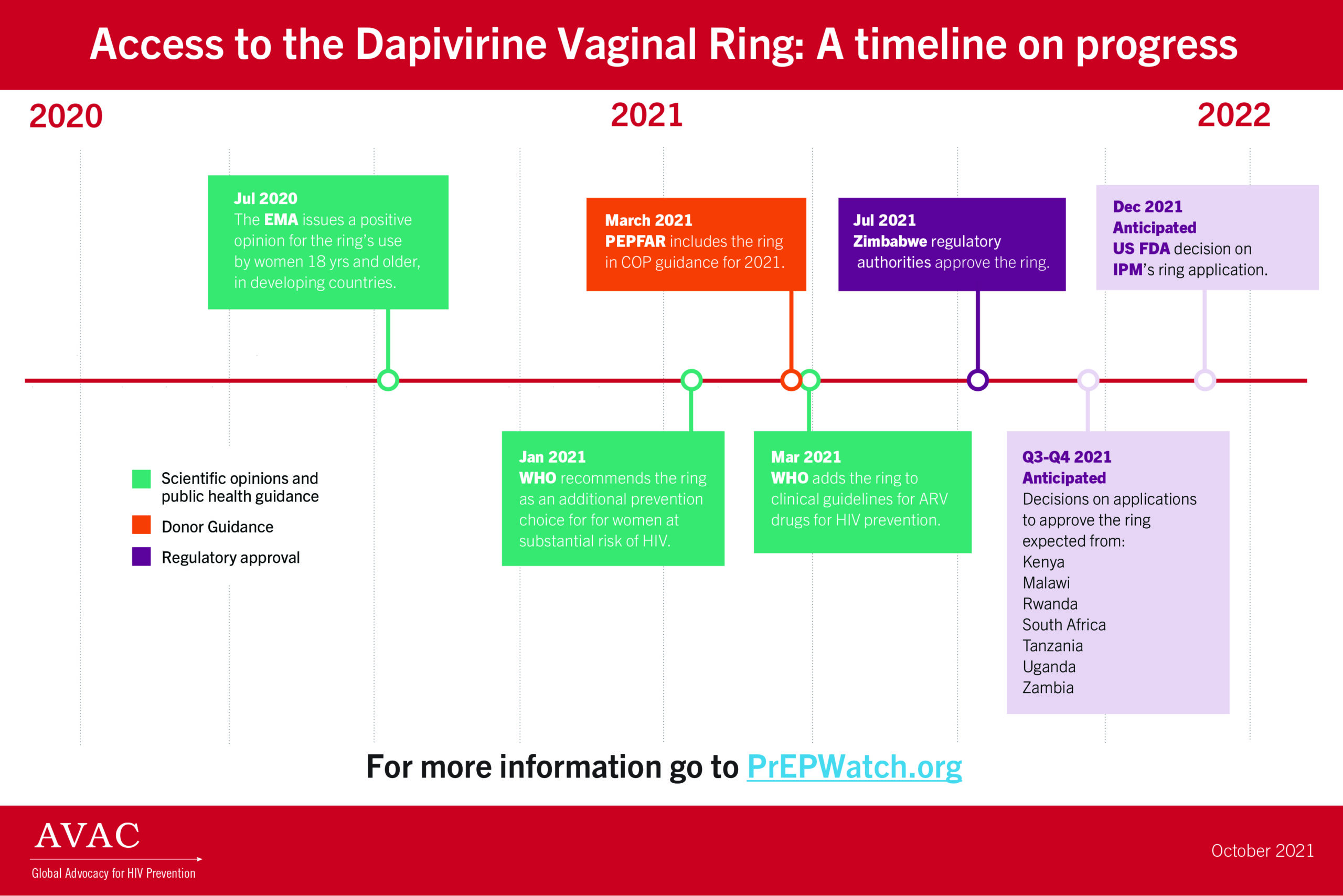
Dapivirine Vaginal Ring Approved in South Africa
The South African Health Products Regulatory Authority (SAHPRA) approved the monthly dapivirine vaginal ring for use by women to reduce their HIV risk. South Africa joins Zimbabwe as the first countries to announce approval of the ring, which is already recommended by the World Health Organization as an additional prevention option. The ring’s developer, the International Partnership for Microbicides, has also submitted additional applications for review by several other Eastern and Southern African countries, and advocates are actively engaged in making the ring accessible, including the fabulous new EmpoweRing campaign from our colleagues at ICWEA. AVAC’s Nandi Luthuli told Herald Live, “we know that the most effective intervention is the one someone picks for themselves among an array of effective choices.”
Updates on Injectable Cabotegravir for PrEP
Following US FDA approval of injectable cabotegravir for PrEP in December, there has been a cascade of activities—and a huge need for advocacy! Multiple additional regulatory agencies are reviewing the application; WHO convened their Guidelines Review Group meeting earlier this month, and guidelines are expected mid-year; and Unitaid announced funding for the first two implementation science projects to introduce injectable PrEP, in Brazil and South Africa. A key lesson from oral PrEP over the past decade has been the essential role of civil society; and advocates released a number of important statements—AfroCAB released two sign-on statements: Communities demand ViiV/GSK accelerate access to CAB-LA in LMICs and ViiV continues to not meet our demands to ensure CAB-LA is accessible for our communities, and a leading group Southern African women’s health advocates released a statement by Southern African Women Advocates in Advance of ViiV Convening. Stay tuned for our updated call to action and roadmap to ensure injectable PrEP and the ring get introduced faster and more strategically.
In Vaccines
New HIV Vaccine Study Using mRNA Platform Launched
The NIH also announced a new study to test three mRNA-based HIV vaccine candidates. This study follows an announcement in January from IAVI about another mRNA-based HIV vaccine study. AVAC is preparing a suite of materials on the latest in HIV vaccine research, development and advocacy for HIV Vaccine Awareness Day in May, so stay tuned. In the meantime, check out this snapshot that compares the two studies.
In COVID
New Resources for Journalists
The COVID-19 pandemic spawned an infodemic inside the onslaught of COVID-related information. Journalists struggle to identify reliable information in the everchanging pandemic landscape. With support from the Rockefeller Foundation, AVAC is expanding our partnership with Internews and our Media Cafe program conveners to support journalists covering HIV prevention science to include reporting on COVID. Check out the curated resources to help journalists find high-quality, understandable information.
WEBINAR: COVID-19 GPP Resources, 6 April at 9am ET/3pm SAST
While there are some resources that address stakeholder engagement in COVID-19 research, they may not adequately reflect the needs of advocates. Join AVAC and partners for a conversation to discuss advocates’ needs and shape the development of future resources. Register here.
WEBINAR: New COVID-19 Vaccines Trials in Sub-Saharan Africa, 6 April at 1oam ET/4pm SAST
Join us for a special webinar on the Ubuntu trial, hosted by the COVID Advocates Advisory Board (CAAB) and our Coalition to Accelerate and Support Prevention Research (CASPR). Led by the COVID-19 Prevention Network (CoVPN), Ubuntu is a new, landmark COVID-19 vaccine trial in sub-Saharan Africa investigating the efficacy of mRNA vaccines in people living with HIV against the omicron variant. Register here.
In Cure
WEBINAR: Breaking Down the Latest in HIV Cure Research, 5 April at 11am ET/5pm SAST
At CROI 2022, a number of exciting updates on HIV cure research were announced, including the most recent case of HIV remission after a stem cell transplant. Join a conversation with researchers as they break down the recent case of HIV cure, which is the first such cure in a woman. Speakers will also provide updates from an ongoing trial studying pediatric remission. Join to learn what these advances mean for science and for people living with HIV. Register here.
In Integration of Sexual Reproductive Health and HIV
WEBINAR: Consultation on the Dual Prevention Pill, 12 April at 8:30am ET/2:30pm SAST
Join FP2030 and AVAC for a conversation about The Dual Prevention Pill (DPP), a daily oral pill that is currently being developed for the simultaneous prevention of unintended pregnancy and HIV acquisition. This consultative webinar will highlight unique perspectives from stakeholder in the fields of family planning and sexual & reproductive health. Register here.
Just Published: Catalyzing action on HIV/SRH integration: lessons from Kenya, Malawi, and Zimbabwe to spur investment
We’re excited to announce this new publication – Catalyzing action on HIV/SRH integration: lessons from Kenya, Malawi, and Zimbabwe to spur investment – in the Global Health Action journal. This publication builds on the partnership between our HIV Prevention Market Manager project, ministry of health officials in Kenya, Malawi and Zimbabwe, and Georgetown University’s Center for Innovation in Global Health, and is a call to catalyze actions by development partners in support of national strategies to integrate HIV and SRH information and services.
We hope these resources offer you the context and tools you need to use your passion and add your voice to the work ahead.