DVR supply available to low- and middle-income countries as of October 2025. For product approvals, volumes, implementation, and price comparisons of long-acting PrEP, visit our dashboard on PrEPWatch.org.
Dapivirine Vaginal Ring Volume
AVAC Applauds Agreements to Accelerate Market Development for Lenacapavir for PrEP
New York, NY, September 24, 2025 — AVAC welcomes parallel announcements from the Gates Foundation and Unitaid on strategic investments to accelerate the development of, access to and price reduction for generic versions of injectable lenacapavir (LEN), the highly effective six-monthly injection for HIV PrEP.
“These investments are a vitally important step in translating the remarkable science of LEN into public health impact,” said Mitchell Warren, executive director of AVAC. “With these agreements, injectable LEN for PrEP gets closer to the price of daily oral PrEP, $40 per person per year, for low and middle-income national governments. This means national programs in many, but not all, countries can begin planning for 2027, at which time ongoing oral PrEP and LEN use will be available at similar prices, meaning many countries will be truly able to offer people who need prevention choice when it comes to the PrEP method that best meets their needs. This could be a transformational moment in HIV prevention if political will, coordination, and further procurement investment meet this moment to deliver LEN with speed, scale and equity to all communities and populations who need and want prevention options. Many questions remain, but in this current environment, we need to seize opportunities and good news when we can.”
These new commitments to accelerate access to generic versions of LEN come on the heels of the Global Fund and PEPFAR re-committing to their December 2024 announcement of reaching two million people with LEN for PrEP within three years, with drug supplies coming from the originator company, Gilead Sciences. Among the outstanding questions from these new commitments is the price of the required oral loading dose for LEN, which is needed to achieve high efficacy. While the cost of ongoing use of LEN for PrEP would be similar to the cost of a year of daily oral PrEP, anyone initiating or restarting LEN for PrEP needs an oral loading dose of LEN. This oral loading dose is not included in the $40 price mentioned in the Gates and Unitaid deals and will add between $15-$17 in the first year of anyone initiating or re-starting LEN, and supply chains and purchasers need to include this extra cost in their calculations.
“The ‘two million in three years’ ambition from Global Fund and PEPFAR must be seen as a floor and not a ceiling,” said Warren. “The global PrEP data that AVAC tracks show a more ambitious goal, getting LEN to at least 1.5 million next year alone, is achievable and necessary. Ultimately, LEN must reach more than five million people per year to have real impact, build a sustainable market, and drive prices down even further. This means we must act faster and think bigger.”
AVAC calls on all stakeholders to do their part. Next steps require coordinated action and further investment to ensure the creation of a viable and sustained market.
“This is the moment to ensure that LEN for PrEP lives up to its full potential, and to hold each other accountable for what must happen next,” said Wawira Nyagah, AVAC’s director of product introduction & access. “Demand creation and program design for LEN must be fully resourced, evidence-based and community-centered. Volume commitments, manufacturing, and supply chains must be sustained and stable. But to make a difference at a global level, the HIV response must go beyond these essential, but minimum, steps with a bold vision to accelerate the entry of generics and trigger a virtuous cycle of price drops, which further drive-up PrEP use.”
LEN, developed by Gilead Sciences, is a twice-yearly injectable PrEP option that showed nearly complete protection against HIV in the landmark PURPOSE 1 and 2 trials. Science Magazine named LEN the “Breakthrough of the Year” in 2024, a recognition that reflects its enormous potential. But fulfilling this potential is far from certain, and all stakeholders have critical work to do, as detailed in AVAC’s 2024 publication, Gears of Lenacapavir for PrEP Rollout.
AVAC’s publication, Now What with Injectable LEN for PrEP How to Translate Ambition into Accelerated Delivery and Impact, includes forecasts demonstrating that instead of 2 million people in three years, the world could reach at least 1.5 million people in just one year. Gilead has confirmed that they can manufacture enough injectable LEN to reach in excess of 5 million LEN users over the next three years. These numbers suggest what is possible and this is no time to think small.
“To achieve true impact against HIV requires early commitments from additional donors to procure large volumes of LEN, which will enable a bigger rollout, exceeding targets, and reaching more people who need PrEP in more places, which in turn secures the kind of market scale that accelerates further prices reductions,” said Nyagah. “It requires country regulators, ministries of health, implementers, advocates and communities where HIV prevention is needed to prepare with policies and programs that will succeed in connecting people with products that work in the context of their lives. The field has learned these lessons before. Technology alone gets you nowhere; it’s delivering the product with speed, scale and equity that gets the job done.”
- Now What with Injectable LEN for PrEP publication and infographic
- Gears of Lenacapavir for PrEP Rollout, publication
- Potential Demand for LEN, infographic
- Philanthropies Strike a Promising Deal to Turn Back H.I.V., New York Times
- Two drugmakers will sell the 6-monthly anti-HIV jab for the price of the daily HIV prevention pill, Bhekisisa
- New partnerships bring price parity between lenacapavir and oral PrEP, Devex
Global Advocates Welcome the Launch of Merck’s EXPrESSIVE Program
Known as the EXPrESSIVE program, the drug maker Merck has two trials testing a monthly pill for PrEP. Merck is committed to stakeholder engagement, putting global advocates at the forefront of planning for the program. Numerous organizations and advocates commend Merck’s dedication to hearing from the community and shared this statement.
The EXPrESSIVE Trials Test a Monthly Pill for PrEP: Advocates speak
The drug maker Merck’s recent announcement at IAS 2025 of two new efficacy trials of a monthly PrEP pill, known as the EXPrESSIVE program, is welcome news. A long-acting PrEP pill would offer a unique new option that could transform the field, contributing significantly to expanding use of HIV prevention, especially to young women, key populations, and those navigating stigma, clinic fatigue, or other barriers to health services.
In addition, Merck’s robust commitment to stakeholder engagement to date contributes an important model of Good Participatory Practice (GPP) to the field, by putting global advocates at the forefront of planning for the program. Merck has expressed a commitment to sustain this vital engagement throughout the program and next steps, and advocates will be holding them to it. AVAC is pleased to share this statement, Global Advocates Welcome the Launch of Merck’s EXPrESSIVE Program, from numerous organizations and advocates who commend Merck “for this important investment in innovation, equity, and choice.”
“This is not just another trial; it’s a signal that the needs of young women and other key groups most affected by HIV really matter,” said Chilufya Kasanda Hampongo, Chair of the Young Women’s HIV Prevention Council (YWHPC). “A monthly pill will offer a new kind of freedom—something discreet, something manageable, something we can own on our terms.”
AVAC has produced several resources to explain and contextualize the EXPrESSIVE program and the monthly PrEP pill.
- Next Up: A monthly pill for PrEP?: This new episode of AVAC’s PxPulse podcast features MSD’s Distinguished Scientist Rebeca Plank and AVAC’s Regional Manager for Research Engagement Grace Kumwenda. They explain the trials’ design, why a monthly pill could be so important to HIV prevention and how GPP is shaping both R&D and rollout of the trials.
- EXPrESSIVE Phase 3 Trials is a new infographic showing the 17 countries hosting trial sites for the two efficacy trials.
- Go to PrEPWatch.org’s dedicated page on MK-8527 for background information on the product and details on the EXPrESSIVE trials.
Now is the time for advocates to serve as both watchdogs and champions for the EXPrESSIVE program and additional options! Join AVAC in tracking this important development in prevention research.
Next Up: A monthly pill for PrEP?
The drug maker Merck has just announced their plans to start two new trials, known as the EXPrESSIVE program, testing a monthly pill for PrEP. A once-a-month PrEP pill holds great potential for the field. Even with daily pills, a monthly ring, or long acting injectables such as cabotegravir and recently FDA-approved lenacapavir, there’ll be people who can’t find what they really need for prevention.
For advocates who follow prevention, there’s a lot to know about these trials, and powerful lessons to learn about Good Participatory Practice (GPP) and impactful involvement of stakeholders—especially community—in research. GPP has been a cornerstone of the process of design and protocol development for the EXPrESSIVE trials, and it doesn’t stop there.
This episode features MSD Distinguished Scientist Rebeca Plank and AVAC’s Regional Manager for Research Engagement Grace Kumwenda. They explain why a monthly pill could be so important to HIV prevention and how GPP is shaping the design and rollout of the trials.
Watch or Listen
Resources
- Global Advocates Welcome the Launch of Merck’s EXPrESSIVE Program, Community Statement
- People’s Research Agenda: Community-Driven Priorities for HIV Prevention Research & Development, AVAC
- Good Participatory Practice (GPP) Guidelines, AVAC
- GPP Body of Evidence: Advocating for Good Participatory Practice (GPP) to become an international standard that makes clinical trials more equitable, just and actionable, AVAC
- Better Engagement, Better Evidence, Lancet
- Learn more about MK-8527 on PrEPWatch.org, AVAC
Now What with Injectable LEN for PrEP?
The announcements on 9 July 2025 from Global Fund and Gilead about their next steps for injectable lenacapavir (LEN) for PrEP are welcome, as one more part of the process. But they raise as many questions as they answer. This brief summary is intended to help outline what is actually known – and not – and what needs to happen next.
Avac Event
Re-Imagining Prevention: Ensuring sustainable PrEP access in an evolving funding context
If you are attending the International AIDS Society Conference (IAS), be sure to join AVAC and the Ministry of Health, Zambia for this vital conversation.
PxWire Volume 15, Issue 3
With the recent FDA approval of injectable lenacapavir (LEN) for PrEP and the drastic withdrawal of US investment in HIV prevention, the field must reimagine and recommit to getting PrEP rollout right this time AND to sustaining the HIV research pipeline. Research on HIV has brought numerous advances to global health, but controlling, and ultimately ending, the epidemic depends on continued investment in innovation.
This issue of PxWire looks at the scale of shuttered prevention programs for key populations (KPs), the potential market for injectable LEN, and the devastating cuts to research for an HIV vaccine.
Read below or download the PDF version of this issue.
Progress in PrEP Uptake
The PEPFAR stop work orders issued by the US government in January 2025 have devastated the HIV response worldwide, including funding for primary prevention. Sustaining the HIV response and bending the curve of incidence depends on identifying new sources of funding to maintain HIV prevention programs for KPs.
- Guidance issued in February 2025 indicated that PrEP services funded by the US government are permitted only for pregnant and lactating people—meaning that KPs, such as LGBTQ+ individuals, sex workers, and people who use drugs, have lost access to PrEP, unless they are currently pregnant or lactating.
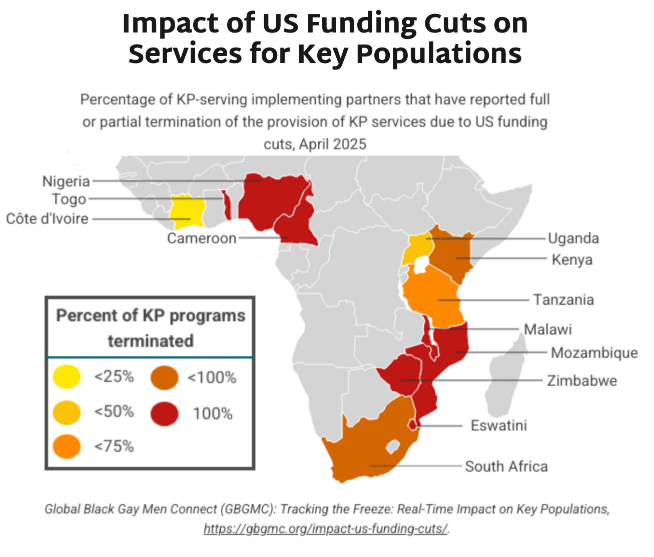
- The graphic below shows key findings on the percentage of KP programs terminated by country. Most of the priority African countries for the HIV response report national KP programs are fully or partially terminated.
- HIV incidence rates amongst KPs are higher than in other populations. Excluding this group from PrEP programming endangers whole communities threatened by HIV, disables the HIV response, and jeopardizes gains made against the epidemic.
- Research undertaken by Global Black Gay Men Connect (GBGMC) examines the impact of US government funding cuts on KP programs in Africa.
- Additional research done by AVAC, in the report Impact of PEPFAR Stop Work Orders, shows that KPs are the group most impacted by US government funding reductions to HIV prevention services worldwide. In some cases, such as Panama, national governments are stepping in to fill the gap.
PrEParing for New Products
Stakeholders—including Global Fund, PEPFAR, WHO, UNAIDS, Unitaid, Ministries of Health, advocates and implementing partners—have critical work to do now to ensure doses of LEN hit the ground as quickly as possible. Check out Gears of Lenacapavir for PrEP Rollout and Getting PrEP Rollout Right This Time to get the details.
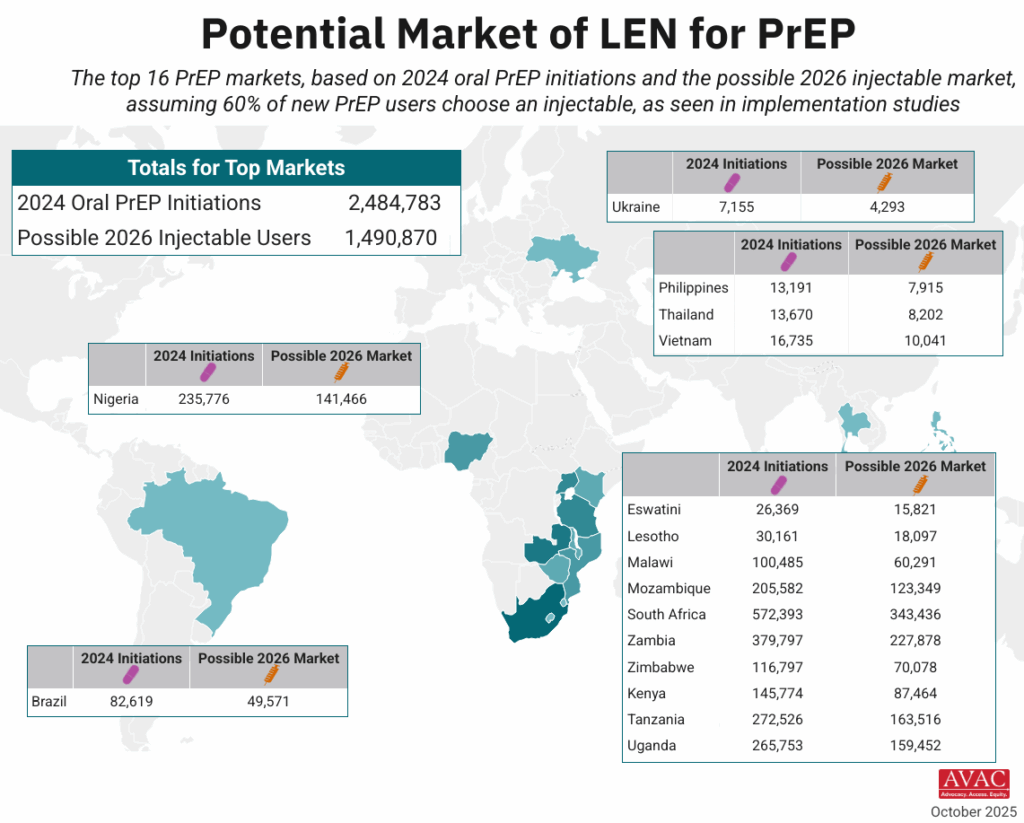
- The map shows 16 countries in Africa, Asia and Latin America that have the largest PrEP markets.
- If total PrEP initiations continue to increase by 20% every year, which is the trend in recent years, and injectables represent 60% of initiations, as seen in implementation studies, 2026 numbers of injectable initiations could be as shown in the map.
- The exact price and volumes of LEN per country is not yet known.
- Of those injectable initiations, LEN is expected to be the majority, given the proposed manufacturing projections from Gilead and the stated ambition of the Global Fund to reach two million people with LEN within the first three years of introduction. Additional volumes of injectable cabotegravir would make up the rest.
- PEPFAR, which in December committed to collaborate with Global Fund, has not yet publicly stated how LEN will fit into their more limited approach to PrEP, which has been restricted to pregnant and breastfeeding women.
The Latest R&D in the Prevention Pipeline
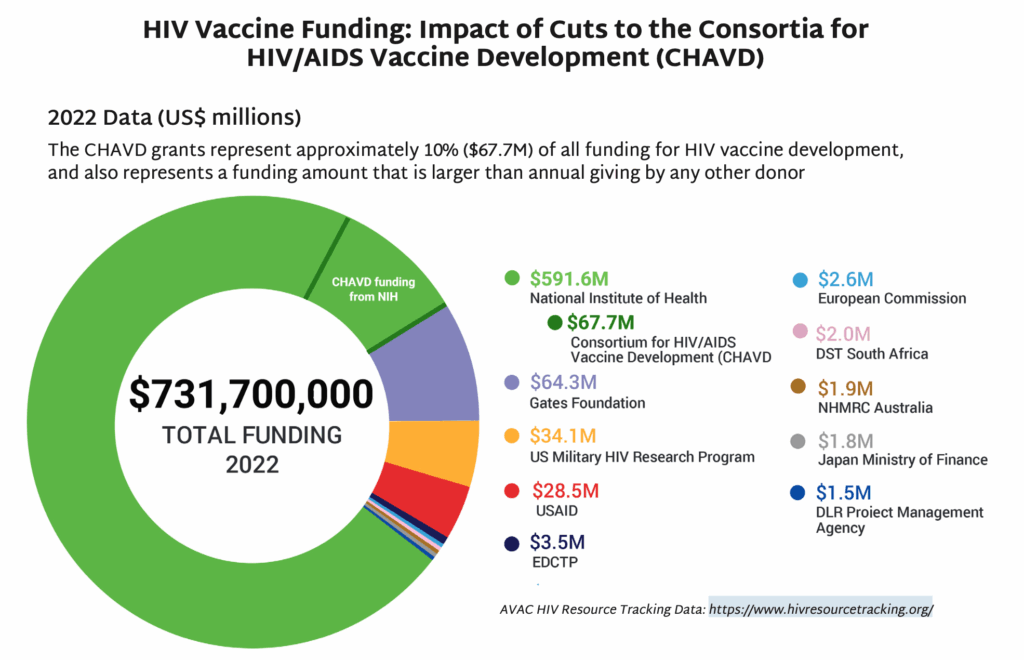
- In May, NIH’s National Institute of Allergy and Infectious Diseases (NIAID) announced that funding for the Consortia for HIV/AIDS Vaccine Development (CHAVD) would end after the current grant cycle in June 2026. With only one more year of funding before the grants end, current plans for research, clinical trials and progress toward a vaccine are all at risk.
- First launched in 2005, the CHAVDs led ground-breaking research to develop an HIV vaccine.
- CHAVD grants currently fund two institutions as consortia leaders—The Scripps Research Institute and Duke University.
- The quest for an HIV vaccine is gaining momentum with field-changing contributions from the CHAVDs. The institutions are currently researching vaccine designs that rely on the immune system’s broadly neutralizing antibodies (bNAbs) to protect against HIV.
- The annual funding for the consortia—approximately $67M—represents a significant chunk of the NIH’s funding for HIV vaccine development, and also approximately 10% of all funding for HIV vaccine research globally each year.
- NIH’s yearly grant total for the CHAVD is larger than any other individual donor’s annual giving for HIV vaccine research. The next closest donor is The Gates Foundation, which donates approximately $64M a year to this research.
- In 20 years of research, CHAVD discoveries have resulted in new technology to combat HIV, influenza, Zika, COVID, and other novel coronaviruses. The loss of the CHAVD will have a devastating impact on the HIV response and scientific discovery.
Prevention Playlist
AVAC develops a wide range of resources to inform decision making and action. Check out the latest:
Join
- CHANGE: In response to the unfolding crisis, more than 1,500 people from civil society organizations around the world have launched CHANGE—Community Health & HIV Advocate Navigating Global Emergencies—a coalition formed to support urgent action: [email protected]
- Subscribe to Global Health Watch: AVAC’s weekly newsletter dedicated to breaking down critical developments in US policies and their impact on global health, at avac.org/global-health-watch
- Fight For Our Lives” Emergency Townhall: Impact of the Trump Administration Foreign Aid Freeze on KP & LGBTQ Communities, Ongoing convening, Register here
Use
- Advocacy Resources for Injectable LEN, AVAC
- HIV Prevention R&D at Risk, AVAC
- Impact of PEPFAR Stop Work Orders, AVAC
- Research Matters Advocacy Toolkit, AVAC, TAG, HIVMA
- Advocates’ Guide: Understanding the President’s Proposed Fiscal Year 2026 (FY26) Budget and Its Implications for Science, Research and Global Health, AVAC
Watch/Listen
- FDA Approves Injectable LEN for PrEP
- Fight for Firewalls: HIV and Health Data Privacy in the Snowballing Surveillance State, Webinar
- Embracing Task Shifting and Innovation to Support Expanded Access to Long-Acting Injectable PrEP, Webinar
- The Scientific Journey of Lenacapavir: From basic science to clinical development to impact, Webinar
- Critical Advocacy: How Civil Society is defending the HIV Response and Global Health, Podcast
- HIV Prevention at a Crossroads: Why we still need an HIV vaccine, Webinar
- A New Era of HIV Prevention: Accelerating access to long-acting prevention options through sustainable prevention systems and financing, Webinar
Read
- FDA Approves Injectable Lenacapavir for PrEP, AVAC
- Trump Aid Cuts Deal a Blow to HIV Prevention in Africa, Reuters
- Will long-lasting HIV preventive be a game changer—or a missed opportunity?, Science
- Regulators Approve a Twice-Yearly Shot to Prevent HIV Infection, New York Times
- FDA Approves Twice-Yearly Lenacapavir for HIV Prevention, POZ
- FDA approves twice-yearly shot for HIV prevention, Helio
- BREAKING: FDA approves breakthrough drug that reduces risk of contracting HIV by 96 percent, The Advocate
- Getting PrEP Rollout Right This Time: Lessons from the field, AVAC
- Why STI Funding Matters, AVAC
- AVAC Condemns Removal of the Advisory Committee on Immunization Practices, AVAC
- AVAC Denounces White House Effort to Codify DOGE Cuts to Health, Research and Foreign Assistance, AVAC
- Protect Federal Funding for HIV, TB, and STI Research and Prevention at the National Institutes of Health, AVAC
- The People’s Research Agenda, AVAC
- Worldwide Prevention, Shared Protection: Why STI Funding Matters, AVAC
Potential Demand for LEN for PrEP
The top 16 PrEP markets, based on 2024 oral PrEP initiations and the possible 2026 injectable market, assuming 60% of new PrEP users choose an injectable, as seen in implementation studies.
Press Release
Important Win for Public Health: Supreme Court Upholds ACA Preventive-Care Protections
Contact: [email protected]
New York, NY, June 27, 2025 — AVAC welcomes today’s ruling affirming the constitutionality of the Affordable Care Act’s preventive services mandate, including coverage for HIV pre-exposure prophylaxis (PrEP) at no cost to patients. This decision represents a critical victory for public health, health equity, and the millions of people who rely on preventive services to stay healthy and safe. By rejecting efforts to strip away access to PrEP and other essential services based on ideological objections, the court has reaffirmed that public policy must be grounded in science, not stigma.
Since the original Braidwood decision, AVAC and our partners have worked tirelessly to raise awareness of the case’s far-reaching implications. We joined legal advocates, public health experts, and community leaders to underscore what was at stake: access to evidence-based care and decades of progress in preventing HIV and other serious conditions. Today’s ruling confirms the power of coordinated advocacy and the importance of protecting science-driven health policy from politically motivated attacks.
This outcome ensures that individuals can continue to access PrEP, both the medication and the clinical services necessary to support its use, without cost barriers. It preserves critical public health gains and sends a strong message that discrimination has no place in our health care system.
“This ruling is a relief in maintaining the critical role under the Affordable Care Act to cover preventive care services, including HIV pre-exposure prophylaxis (PrEP),” said Mitchell Warren, AVAC’s Executive Director. “Preventive services across healthcare are cost-saving and life-saving, and I am grateful that the Supreme Court found on the side of evidence, logic, public health, and human rights. There has been enormous progress in the fight to end the HIV epidemic, and just last week the FDA approved the newest form of PrEP, injectable lenacapavir. Lenacapavir can be a transformative option, but only if it is available to people who want and need it, and today’s ruling can make that possible.”
Looking ahead, AVAC will continue working to ensure that PrEP access is not only protected but meaningfully expanded, particularly for the communities that have long faced systemic barriers to care. This includes advocating for a national PrEP program, strengthening provider and patient education, supporting implementation by community-led organizations, and holding insurers accountable for compliance. Today’s ruling offers a strong foundation to build from, and we remain committed to a future where HIV prevention is accessible, equitable, and fully resourced for all.
Today’s Supreme Court decision does confirm enormous power with the Secretary of Health and Human Services, which under the current administration is cause for significant concern. “In the midst of today’s victory, we must be tempered by what has happened with the CDC’s Advisory Committee on Immunization Practices (ACIP), as it could be a harbinger of what a Secretary of HHS can do to twist committees and task forces that should be composed of technical experts guided by science to ones that are guided by ideology, illogic and political whim,” said Warren.
###
About AVAC: Founded in 1995, AVAC is an international non-profit organization that provides an independent voice and leverages global partnerships to accelerate ethical development and equitable delivery of effective HIV prevention options, as part of a comprehensive and integrated pathway to global health equity. Follow AVAC on Bluesky and Instagram. Find more at www.avac.org and www.prepwatch.org.