Join AVAC and partners for webinar on May 3 webinar, 10-11:30am EDT where you can engage with researchers who led the studies about this injectable PrEP strategy and advocates who are leading essential advocacy efforts around the introduction of CAB-LA. On the call, lead trial investigators Sinead Delany-Moretlwe from HPTN 084 and Raphy Landovitz from HPTN 083 will provide updates, and we’ll be joined by AVAC’s Emily Bass, Chiluyfa Kasanda from TALC in Zambia, Richard Lusimbo from Pan Africa ILGA, and Sibongile Maseko who is an independent consultant and women’s health advocate based in Eswatini. Register here.
Long-Acting Injectable Cabotegravir for PrEP: Understanding Results of HPTN 083 & 084 and key areas for advocacy
Moving Ahead with Long-Acting PrEP: Webinar and updated resource on CAB-LA research and advocacy
Long-acting injectable cabotegravir (CAB-LA) for PrEP is causing buzz and raising opportunities and questions for prevention advocates. Whether you’ve got questions or want to know what the buzz is about, AVAC has you covered.
We’ve updated our comprehensive primer on CAB-LA: Advocates’ Primer on Long-Acting Injectable Cabotegravir for PrEP: Understanding the Initial Results of HPTN 083 and HPTN 084, and held a May 3 webinar, 10-11:30am EDT where you can listen to the researchers who led the studies about this injectable PrEP strategy and advocates who are leading essential advocacy efforts around the introduction of CAB-LA.
On the call, lead trial investigators Sinead Delany-Moretlwe from HPTN 084 and Raphy Landovitz from HPTN 083 provided updates, and we were joined by AVAC’s Emily Bass, Chiluyfa Kasanda from TALC in Zambia, Richard Lusimbo from Pan Africa ILGA, and Sibongile Maseko who is an independent consultant and women’s health advocate based in Eswatini.
Watch the recording and find the slides here.
As our new primer describes, CAB-LA injections every eight weeks provided high levels of protection against HIV in cisgender women, cisgender men who have sex with men and transgender women who have sex with men. That’s truly exciting. There’s also a lot to learn and understand about next steps. A small number of people who received on-time injections and went on to acquire HIV did not test “HIV-positive” on standard antibody-based HIV tests. An even smaller number acquired resistance to integrase inhibitors—the class that includes cabotegravir and dolutegravir. In addition to these crucial issues, it’s also important to focus on questions of access. For those who want an injectable PrEP strategy, CAB-LA needs to be accessible and affordable.
What do the trial results explain, what still needs to be explored, and what do advocates think needs to happen next? Check out the updated resource and register the webinar to engage with it all!
New Resources on AVAC.org
AVAC has several new resources covering a gamut of cutting-edge issues for the field. An up-close look at the science covered at CROI; a handy snapshot of multipurpose technology (MPTs) moving through the research pipeline; a new infographic on “time to market” for HIV prevention products furthest along in development; and a special publication of Good Participatory Practice fitted to address COVID-19 trials. Read on for details and links for these timely resources.
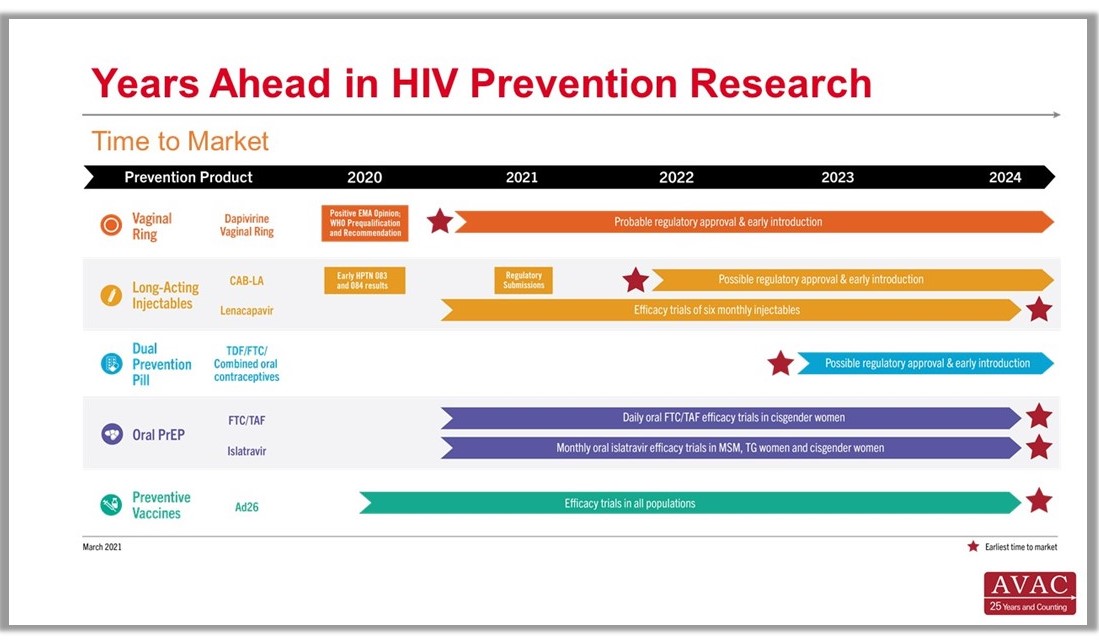
Time to Market Infographic
Years Ahead in HIV Prevention Research: Time to Market – The latest addition to our extensive infographic library is this new timeline showing the potential time points when the next-generation of HIV prevention options might find their way into new programs. This new graphic complements the HIV Px Research, Development and Implementation pipeline snapshot and The Years Ahead in Biomedical HIV Px Research trials timeline.
MPTs Making Headway
Advocates’ Guide to Multipurpose Prevention Technologies – Check out this guide to learn about four areas ripe for advocate involvement and get a snapshot on the status of MPT research and development, and data on investments.
Good Participatory Practice in the Age of COVID-19
Essential Principles & Practices for GPP Compliance: Engaging stakeholders in biomedical research during the era of COVID-19 – This guide to support stakeholder engagement in COVID-19 research is built from the Good Participatory Practice Guidelines for Biomedical HIV Prevention Trials (GPP). This new document responds to needs expressed by both researchers and advocates as COVID-19 research progresses with unprecedented speed and urgency. To mark the launch of this new document, AVAC hosted a webinar earlier today, which included diverse perspectives on the importance of GPP within COVID-research and beyond. Watch the recording here.
CROI in Focus
The Personal is Planetary: CROI and COVID one year on – This blog by AVAC’s Emily Bass gives context and perspective on the science and advocacy that defined the Conference on Retroviruses and Opportunistic Infections in 2021. From a call for vaccine equity to a deep dive into the findings on cabotegravir as long-acting injectable PrEP, read Bass’s blog for a picture on where the science and advocacy is moving.
We are also happy to report that, in response to community requests, CROI organizers have agreed to make all recorded content from the meeting available on April 15—five months earlier than initially planned. And, if you missed it, check out the recordings from the Daily Research Updates for advocates on AVAC’s special CROI page.
New Resource! Advocates’ Guide to Multipurpose Prevention Technologies
AVAC has a new resource to support advocacy for multipurpose prevention technologies (MPTs), Advocates’ Guide to Multipurpose Prevention Technologies. The Guide calls out four areas ripe for advocate involvement. It also provides a snapshot on the status of MPT research and development and data on investments—critical information that can support evidence-based advocacy.
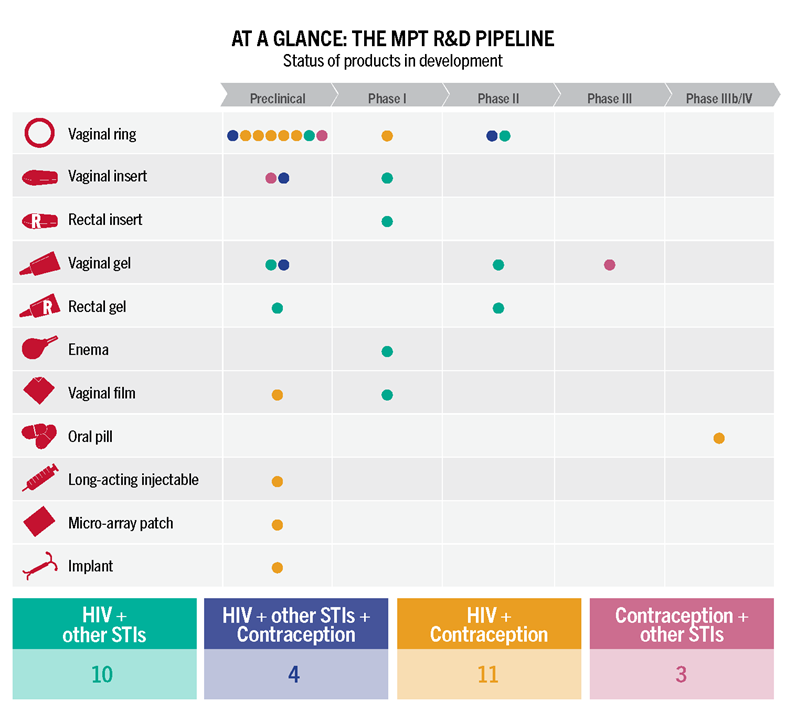
MPTs are products designed to simultaneously address more than one sexual and reproductive health (SRH) concern. Male and female condoms—which protect against pregnancy as well as HIV and other STIs—are the only MPTs available today. MPTs offer great promise for meeting people’s diverse and changing SRH needs. But advocacy for these emerging technologies is crucial to mobilize resources and broaden support through all stages of MPT R&D to ensure equitable access to products as they become available.
This new resource complements AVAC’s ongoing work to advance the integration of services for sexual and reproductive health (SRH) with HIV treatment and prevention. For the latest on the Dual Prevention Pill (DPP)—the next MPT that is likely to go to market soonest—visit its dedicated page on PrEPWatch.
AVAC is also supporting a pioneering initiative led by the Ministry of Health in Kenya, jointly run by NASCOP and the Department of Family Health, to work with implementing partners, county-level officials, advocates and other stakeholders, to integrate HIV prevention and SRH policies and services. Check out the assessment that preceded this important work in Kenya and a similar assessment with partners in Zimbabwe. Watch this space for progress as it develops, and bookmark www.srhintegration.org and www.avac.org/advance-hiv-srh-integration for an overview of the issues and key resources.
The Personal is Planetary: CROI and COVID one year on
Emily Bass is AVAC’s Director of Strategy & Content.
Last week, CROI went virtual as the world marked a year since the World Health Organization officially declared COVID-19 a global pandemic. Like everyone else, I had my own personal milestones from that week last year. I remember a dear friend who’d traveled from Uganda for CROI 2020 sitting in my living room, worrying about whether to attend the event before it pivoted to virtual at the last minute. I remember her deciding to leave and embracing her—unmasked—on the sidewalk in front of my house when she did. I do not have any idea now when we will see each other again.
A pandemic happens to a planet, but it is measured in individuals. Holding both of those realities at the same time is the challenge, and at this year’s CROI, no one did it better than Fatima Hassan and Gregg Gonsalves who shared a screen and billing, as joint presenters of the esteemed Martin Delaney Lecture. The pair delivered it not as a lecture but a conversation, thinking aloud about pernicious manifestations of vaccine nationalism and the inadequacy of current purchasing arrangements to circumvent a level of medical apartheid in vaccine distribution not seen since the era in which they fought for universal access to antiretrovirals. The conversation was simultaneously intimate and impassioned, which may have been why a comment Hassan made in passing nearly moved me to tears. She said she didn’t expect to see Gregg, a friend for two decades, “for years.”
Hassan and Gonsalves said many critical things in their talk—which CROI has made available (in response to community requests, CROI changed its original policy to keep all 2021 content behind a paywall for six months—all recorded content will now be available on April 15). Their clear, methodical and damning conversation tied today’s pandemic to the early years of HIV/AIDS. Gonsalves quoted irascible, irreplaceable AIDS activist Larry Kramer who said that HIV/AIDS was about “disposable people“, the Black, brown, queer, immigrant and poor people who are not valued by the capitalist state. “Now we see [with COVID] another set of disposable people,” Gonsalves said. “Many of us didn’t think we would have to see this again.”
Immediately after their talk, a working group came together to draft and launch a Call for Vaccine Equity and it was subsequently published in BMJ. It outlined a set of demands including a call for the United States government to incentivize pharmaceutical companies like Pfizer, Moderna and Johnson & Johnson to share knowledge on how to produce their vaccines; World Trade Organization members to waive the trade-related intellectual property (TRIPS) provisions that serve as a barrier to generic production; and COVAX partners to acknowledge that its anticipated vaccine coverage rates of 20 percent of the population of countries dependent on COVAX facilities is simply too low. The cost of inaction will be measured in numbers that sometimes make the mind go numb; it will also be measured in the time that comrades spend separated by continents but bound in the common fight for equity.
This is not a test: Time to tackle HIV diagnostics, counseling messages, pricing and more for PrEP in its many forms
CROI brought new information on long-acting injectable cabotegravir (CAB-LA) for PrEP, which has previously shown high levels of efficacy in reducing risk of HIV in gay men and other men who have sex with men and transgender people who have sex with men in a trial called HPTN 083; and in cisgender women, in a trial called HPTN 084.
Building on data presented at AIDS 2020, Dr. Raphy Landovitz presented an in-depth analysis of the timing of HIV infection in HPTN 083 participants, relative to when they were diagnosed with HIV, what product they were receiving, and how much of the drug was present in their blood.
A highly-detailed summary of the findings can be found here, in Gus Cairns’ always-excellent coverage for aidsmap. Broadly speaking, there are three key findings that advocates will need to react to and act on as part of a comprehensive PrEP agenda:
1. In people using injectable CAB-LA and daily oral TDF/FTC PrEP, standard HIV tests do not always provide accurate, real-time results. Most HIV tests look for antibodies to the virus and/or antigen (a molecule found on the surface of the virus) from HIV. People who have very low levels of HIV, or who have acquired HIV very recently, do not always have antibodies to the virus—and so they will test “negative” on standard diagnostics. Taking an antiretroviral like CAB-LA can suppress HIV to very low levels for a time, meaning that people who acquire HIV while receiving the injection may not test positive on standard diagnostics.
This was the case for four participants in HPTN 083 who acquired HIV even though they had received shots of CAB-LA as scheduled. These individuals did, eventually, test positive on the standard HIV antibody and antigen tests used at study visits, but when the trial team looked back at blood samples from these four people at prior visits, and used more sensitive laboratory diagnostics, they found that each had acquired HIV weeks, if not months, prior to the first positive test result at the site. Landovitz said there is “a need to look [for HIV] with diagnostics that are appropriately sensitive. And with long-acting agents for prevention [e.g., cabotegravir], currently available diagnostics are blunted or delayed in sensitivity.”
A presentation by Dr. Donn Colby looking at people using daily oral PrEP in Thailand also found eight individuals who had HIV at the time that they initiated oral PrEP but tested negative on standard HIV tests at enrollment. Five of these individuals tested were identified via a pooled RNA test (a group of samples tested for signs of HIV genetic material, which is detectable earlier after infection than antigen or antibody). Three individuals tested positive after starting PrEP. As in HPTN 083, the site went back to look at previously collected samples and found viral RNA when an array of standard tests gave a “negative” result.
The number of people with HIV whose virus isn’t picked up via standard tests is relatively small. Colby estimated about one case of acute infection (the term for the very early phase after HIV acquisition) per 400 people initiating PrEP at the Thai PrEP clinic; in HPTN 083, the four individuals with HIV at baseline (described above) were out of a group of 2,282—which is one per 570.
But even with these small numbers, there are big questions: how should the risk of a false negative be conveyed to people when they start a PrEP strategy like CAB-LA where it appears that there can be a long interval between infection and diagnosis via antibody/antigen tests? What approaches can be used in programs when a person is starting PrEP initiation? Finding people with acute HIV infection allows them to start treatment right away if they choose to do so. It also minimizes the risk that a person with HIV will start single-drug PrEP, which can lead to drug resistance.
Both of the solutions shared at CROI are practical in some ways and present challenges in other ways. In the Thai clinic, people who report a behavior in the past month that is associated with high risk of HIV are initiated on three-drug ART for the first month of PrEP, before a confirmatory negative test allows them to shift from three-drug ART to a single drug for PrEP. Both Colby and Landovitz talked about the need to consider using viral load assays to confirm HIV status. These tests, used to detect HIV in the blood of people living with the virus, are far more sensitive than diagnostics; they’re also far more expensive. Hearing this suggestion, in the context of ongoing gaps in coverage of viral load testing for people living with HIV, immediately made me think of the conversation around oral PrEP in the years following the first evidence of its efficacy. Then, treatment waitlists and shortages of TDF/FTC for people living with HIV made a push for expanded rollout of TDF/FTC for prevention untenable for many activists. Calls to introduce viral load testing as a diagnostic for either CAB-LA, oral PrEP or the Dapivirine Vaginal Ring must happen in the context of a concerted, funded effort to provide universal viral load coverage—at least one viral load test per year—to all people living with HIV. This is by no means a given.
CROI itself featured presentations on approaches that could provide cheap, early alternatives to viral load tests as means of determining when a person’s current HIV drug combination is no longer suppressing her virus. (A detectable viral load is used as a sign of “treatment failure.”) Dr. Jose Castillo-Mancilla presented on the use of dried blood spot tests to detect emtricitabine triphosphate. This form of TDF/FTC is only detectable in the blood within 36 hours after a person has taken a pill; testing for presence or absence is a way of finding out whether a person has recently missed a dose. Castillo-Mancilla and his colleagues found that people with no detectable emtricitabine triphosphate were more likely to have detectable viral loads in the future. Dr. Jacantha Odayar and her colleagues in a South Africa study found that presence or absence of tenofovir diphosphate (another form TDF/FTC takes as it is broken down, or metabolized, in the body) in blood predicted future viremia in pregnant women. Such tests, which focus on TDF/FTC containing regimens, could be used as a “early warning” system for predicting virologic failure among people living with HIV.
2. Counseling needs to catch up to high-tech PrEP. Landovitz’s presentation also suggested that rolling out CAB-LA for PrEP will require counseling strategies for people who test positive while receiving the injections as prescribed. In HPTN 083, one of the people who acquired HIV, despite on-time injections, initially refused to accept the diagnosis in part because standard test results were inconclusive. This individual opted for a month of post-exposure prophylaxis instead of starting treatment. Delays in diagnosis, confounding results on standard diagnostics and individual disbelief in positive test results will all come up in “real-world” use. Counseling and follow-up must be developed with these needs in mind.
A presentation by Dr. Catherine Koss from the SEARCH study offered another reminder that social networks play a key role in PrEP use. The study made a valuable investment in looking at both community, social and structural aspects impacting health behaviors. SEARCH used an elaborate system of contact mapping and concluded that people who knew someone using PrEP were 57 percent more likely to use PrEP themselves. Destigmatizing and normalizing PrEP so that people talk about it when they choose to use it (or stop or restart it), and incorporating peer-to-peer support for all forms of PrEP into programs will likely be key.
Resistance happens among people taking CAB-LA but not necessarily during the “tail”. Long-acting products like CAB-LA include a period, after a person has stopped receiving injections, when the drug is still in the body but at levels that don’t provide protection against infection—often referred to as the “tail”. (For any PrEP strategy, there’s a blood-drug level that’s associated with prevention; you don’t need “some” PrEP drug but “enough” to reduce HIV risk.) There’s been real concern about drug resistance arising in people who acquire HIV during the tail. But in the three people who fell into this category in HPTN 083, none had HIV with genetic mutations that render it resistant to integrase inhibitors, the class of drug that includes cabotegravir. This number is too small to support conclusions about the risk of resistance, but in this handful of people, resistance didn’t emerge during the tail. When integrase inhibitor resistance did emerge in HPTN 083 participants, it was in people who were infected and also receiving the injections: one of the people who had HIV at the time of enrollment developed resistance, as did two people who acquired HIV during the oral lead-in phase (a trial period where individuals took the pill form of CAB-LA before receiving the injection, to ensure that the drug was well-tolerated). Two people who acquired HIV while receiving CAB-LA injections also developed integrase inhibitor resistance; two others had so little virus in their blood that the team could not get a resistance test. The take-home message—at least from this trial—is that the risk of resistance is greater when people acquire HIV while taking CAB-LA as prescribed than it is in people who’ve stopped receiving the injection, i.e., in the context of the “tail”. Cabotegravir is an integrase inhibitor, as is dolutegravir (DTG), which is used as first-line treatment in many settings. The people with integrase-inhibitor-resistant virus responded to alternative regimens, including TDF/FTC-efavirenz-based therapy and a regimen using a boosted protease inhibitor. In resource-constrained settings where countries have fully embraced DTG-based first-line therapy, it will be essential to plan for CAB-LA introduction in the context of treatment options for those with integrase inhibitor-resistance.
3. Injectable PrEP is “superior” to oral PrEP among people who can’t or don’t adhere to the daily pill. Landovitz also presented an analysis of serum and dried blood spot samples from HPTN 083 participants who were assigned to receive daily oral TDF/FTC, the comparison arm of the study. (In each arm, people also received a dummy, or “inert” version of the other product—people randomized to TDF/FTC received saline injections, people receiving CAB-LA injections received dummy pills.) Thirty-seven of the 39 individuals who were prescribed and counseled on the active daily pill and went on to acquire HIV had “suboptimal or non-adherent” levels of the drug in their samples at the time of infection. The study concluded that CAB-LA was “superior” to oral TDF/FTC. These new data clarify that people who received CAB-LA injections on schedule every two months had a substantially lower risk of HIV than people who received daily oral PrEP but did not or were not able to take it as prescribed. In other words, the difference in incidence rates relates to product use, and not the product itself.
One strategy is not inherently superior to the other, i.e., injections and daily oral PrEP each taken exactly as prescribed may well provide comparable levels of protection; the study didn’t evaluate this question.
Products exist in the real world, where people’s lives, circumstances, bodies and preferences play a role in what’s possible and pleasurable. In the real world—as several CROI presentations highlighted—starting and staying on daily oral PrEP is a challenge for some. The 083 data say much the same. “Choice is the answer,” declared the inimitable Dr. Linda-Gail Bekker in her plenary presentation, emphasizing that many oral PrEP programs see people using the strategy with complex patterns of starting and re-initiation. Understanding these patterns of use—and their impact on both individual risk and population incidence—is key to understanding the impact of PrEP.
Time to calculate the cost of progress—and of inaction
CROI also brought updates from product developers working on other types of long-acting PrEP, including a one-year implant containing the drug islatravir that could be placed under the skin for continuous protection, and a Dapivirine Vaginal Ring (DVR) that could be worn for three months. This is three times as long as a monthly ring that has received a favorable opinion from the European Medicines Agency and been recommended by the WHO. The International Partnership for Microbicides is presently submitting the monthly DVR for regulatory approval in a range of countries.
Islatravir (both as an annual implant and as a monthly pill, which is now in efficacy trials), a three-month ring and the six-monthly injectable lenacapavir look promising and would make choice-based programming real by expanding options and allowing prevention programs to meet people where they are. Long-acting PrEP might, for example, play a role in HIV prevention for postpartum or breastfeeding women. A study of PrEP use among pregnant and breastfeeding women found higher PrEP uptake among pregnant women compared to those who were not pregnant, and a drop-off in PrEP use during breastfeeding. But risk remains high for HIV-negative women who are breastfeeding—a reminder that matching prevention offerings with lifecycle events is a life-saving imperative.
All of these offerings will come at a cost, of course. A “superior” strategy like CAB-LA could reduce HIV in many people, but could also require new testing tools and counseling strategies—and still won’t work for everyone, including transgender individuals with buttock implants, unless an alternate injection site can be identified. A 90-day ring and a one-year implant will, similarly, offer transformative options for reducing HIV risk, but will not address rates of STIs that remain very high in many study populations. Transformative PrEP programs will be ones that offer choice in both HIV and contraceptive options, and sexual and reproductive health care, including STI prevention and treatment, for people of all gender identities and sexual orientations. It’s not possible to plan or budget for such programs without knowing the cost of the products themselves. To date, ViiV, the developer of CAB-LA, has not set a price for CAB-LA. A modeling study presented by Dr. Anne Neilan asked the question, “How much should we be willing to pay for CAB-LA for MSM and transgender women, compared to generic daily oral TDF/FTC, or branded FTC/TAF.” Using a set of prices specific to the Global North, Neilan and her colleagues proposed that the injection would need to be priced comparably to generic daily oral PrEP for it to be considered “cost effective”.
While such models and projections always have limitations, they provoke useful and necessary discussions. Will the price be set according to this calculation? If it is not, will gaps in PrEP access open as they once did with antiretrovirals for HIV, and as they have for COVID-19 vaccines? Or will the HIV field, in all its diversity, set an example for how to learn from history and advance equity for the future?
At the end of CROI, I was no more certain of this than when I might see my Ugandan friend again—or when Hassan and Gonsalves might next sit side by side in real life. But I did know, in my bones, that if there was progress towards equity and justice it would come from the human connections between activists, allies and friends that have always transcended physical distance out of love and anger, and a commitment to lasting change.
The Personal is Planetary: CROI and COVID one year on
Last week the Conference on Retroviruses and Opportunistic Infections (CROI) took place online—as the conference went virtual for the second year in a row. There are a number of excellent sources of news to catch all that was shared at the meeting including coverage from aidsmap, HIV-iBase and NATAP. And for recordings of the daily advocate/researcher breakfast updates (or margarita, depending on your time zone or personal preferences) are available at www.avac.org/croi2021. And, of course, there’s AVAC’s take on the news from CROI in a new blog post below. Enjoy!
The Personal is Planetary: CROI and COVID one year on
By Emily Bass
________________________________________
Last week, CROI went virtual as the world marked a year since the World Health Organization officially declared COVID-19 a global pandemic. Like everyone else, I had my own personal milestones from that week last year. I remember a dear friend who’d traveled from Uganda for CROI 2020 sitting in my living room, worrying about whether to attend the event before it pivoted to virtual at the last minute. I remember her deciding to leave and embracing her—unmasked—on the sidewalk in front of my house when she did. I do not have any idea now when we will see each other again.
A pandemic happens to a planet, but it is measured in individuals. Holding both of those realities at the same time is the challenge, and at this year’s CROI, no one did it better than Fatima Hassan and Gregg Gonsalves who shared a screen and billing, as joint presenters of the esteemed Martin Delaney Lecture. The pair delivered it not as a lecture but a conversation, thinking aloud about pernicious manifestations of vaccine nationalism and the inadequacy of current purchasing arrangements to circumvent a level of medical apartheid in vaccine distribution not seen since the era in which they fought for universal access to antiretrovirals. The conversation was simultaneously intimate and impassioned, which may have been why a comment Hassan made in passing nearly moved me to tears. She said she didn’t expect to see Gregg, a friend for two decades, “for years.”
Hassan and Gonsalves said many critical things in their talk—which CROI has made available (an exception to a new and regrettable policy that all 2021 content will remain behind a paywall for six months). Their clear, methodical and damning conversation tied today’s pandemic to the early years of HIV/AIDS. Gonsalves quoted irascible, irreplaceable AIDS activist Larry Kramer who said that HIV/AIDS was about “disposable people”, the Black, brown, queer, immigrant and poor people who are not valued by the capitalist state. “Now we see [with COVID] another set of disposable people,” Gonsalves said. “Many of us didn’t think we would have to see this again.”
Immediately after their talk, a working group came together to draft and launch a Call for Vaccine Equity and it was subsequently published in BMJ. It outlined a set of demands including a call for the United States government to incentivize pharmaceutical companies like Pfizer, Moderna and Johnson & Johnson to share knowledge on how to produce their vaccines; World Trade Organization members to waive the trade-related intellectual property (TRIPS) provisions that serve as a barrier to generic production; and COVAX partners to acknowledge that its anticipated vaccine coverage rates of 20 percent of the population of countries dependent on COVAX facilities is simply too low. The cost of inaction will be measured in numbers that sometimes make the mind go numb; it will also be measured in the time that comrades spend separated by continents but bound in the common fight for equity.
This is not a test: Time to tackle HIV diagnostics, counseling messages, pricing and more for PrEP in its many forms
CROI brought new information on long-acting injectable cabotegravir (CAB-LA) for PrEP, which has previously shown high levels of efficacy in reducing risk of HIV in gay men and other men who have sex with men and transgender people who have sex with men in a trial called HPTN 083; and in cisgender women, in a trial called HPTN 084.
Building on data presented at AIDS 2020, Dr. Raphy Landovitz presented an in-depth analysis of the timing of HIV infection in HPTN 083 participants, relative to when they were diagnosed with HIV, what product they were receiving, and how much of the drug was present in their blood.
A highly-detailed summary of the findings can be found here, in Gus Cairns’ always-excellent coverage for aidsmap. Broadly speaking, there are three key findings that advocates will need to react to and act on as part of a comprehensive PrEP agenda:
1. In people using injectable CAB-LA and daily oral TDF/FTC PrEP, standard HIV tests do not always provide accurate, real-time results. Most HIV tests look for antibodies to the virus and/or antigen (a molecule found on the surface of the virus) from HIV. People who have very low levels of HIV, or who have acquired HIV very recently, do not always have antibodies to the virus—and so they will test “negative” on standard diagnostics. Taking an antiretroviral like CAB-LA can suppress HIV to very low levels for a time, meaning that people who acquire HIV while receiving the injection may not test positive on standard diagnostics.
This was the case for four participants in HPTN 083 who acquired HIV even though they had received shots of CAB-LA as scheduled. These individuals did, eventually, test positive on the standard HIV antibody and antigen tests used at study visits, but when the trial team looked back at blood samples from these four people at prior visits, and used more sensitive laboratory diagnostics, they found that each had acquired HIV weeks, if not months, prior to the first positive test result at the site. Landovitz said there is “a need to look [for HIV] with diagnostics that are appropriately sensitive. And with long-acting agents for prevention [e.g., cabotegravir], currently available diagnostics are blunted or delayed in sensitivity.”
A presentation by Dr. Donn Colby looking at people using daily oral PrEP in Thailand also found eight individuals who had HIV at the time that they initiated oral PrEP but tested negative on standard HIV tests at enrollment. Five of these individuals tested were identified via a pooled RNA test (a group of samples tested for signs of HIV genetic material, which is detectable earlier after infection than antigen or antibody). Three individuals tested positive after starting PrEP. As in HPTN 083, the site went back to look at previously collected samples and found viral RNA when an array of standard tests gave a “negative” result.
The number of people with HIV whose virus isn’t picked up via standard tests is relatively small. Colby estimated about one case of acute infection (the term for the very early phase after HIV acquisition) per 400 people initiating PrEP at the Thai PrEP clinic; in HPTN 083, the four individuals with HIV at baseline (described above) were out of a group of 2,282—which is one per 570.
But even with these small numbers, there are big questions: how should the risk of a false negative be conveyed to people when they start a PrEP strategy like CAB-LA where it appears that there can be a long interval between infection and diagnosis via antibody/antigen tests? What approaches can be used in programs when a person is starting PrEP initiation? Finding people with acute HIV infection allows them to start treatment right away if they choose to do so. It also minimizes the risk that a person with HIV will start single-drug PrEP, which can lead to drug resistance.
Both of the solutions shared at CROI are practical in some ways and present challenges in other ways. In the Thai clinic, people who report a behavior in the past month that is associated with high risk of HIV are initiated on three-drug ART for the first month of PrEP, before a confirmatory negative test allows them to shift from three-drug ART to a single drug for PrEP. Both Colby and Landovitz talked about the need to consider using viral load assays to confirm HIV status. These tests, used to detect HIV in the blood of people living with the virus, are far more sensitive than diagnostics; they’re also far more expensive. Hearing this suggestion, in the context of ongoing gaps in coverage of viral load testing for people living with HIV, immediately made me think of the conversation around oral PrEP in the years following the first evidence of its efficacy. Then, treatment waitlists and shortages of TDF/FTC for people living with HIV made a push for expanded rollout of TDF/FTC for prevention untenable for many activists. Calls to introduce viral load testing as a diagnostic for either CAB-LA, oral PrEP or the Dapivirine Vaginal Ring must happen in the context of a concerted, funded effort to provide universal viral load coverage—at least one viral load test per year—to all people living with HIV. This is by no means a given.
CROI itself featured presentations on approaches that could provide cheap, early alternatives to viral load tests as means of determining when a person’s current HIV drug combination is no longer suppressing her virus. (A detectable viral load is used as a sign of “treatment failure.”) Dr. Jose Castillo-Mancilla presented on the use of dried blood spot tests to detect emtricitabine triphosphate. This form of TDF/FTC is only detectable in the blood within 36 hours after a person has taken a pill; testing for presence or absence is a way of finding out whether a person has recently missed a dose. Castillo-Mancilla and his colleagues found that people with no detectable emtricitabine triphosphate were more likely to have detectable viral loads in the future. Dr. Jacantha Odayar and her colleagues in a South Africa study found that presence or absence of tenofovir diphosphate (another form TDF/FTC takes as it is broken down, or metabolized, in the body) in blood predicted future viremia in pregnant women. Such tests, which focus on TDF/FTC containing regimens, could be used as a “early warning” system for predicting virologic failure among people living with HIV.
2. Counseling needs to catch up to high-tech PrEP. Landovitz’s presentation also suggested that rolling out CAB-LA for PrEP will require counseling strategies for people who test positive while receiving the injections as prescribed. In HPTN 083, one of the people who acquired HIV, despite on-time injections, initially refused to accept the diagnosis in part because standard test results were inconclusive. This individual opted for a month of post-exposure prophylaxis instead of starting treatment. Delays in diagnosis, confounding results on standard diagnostics and individual disbelief in positive test results will all come up in “real-world” use. Counseling and follow-up must be developed with these needs in mind.
A presentation by Dr. Catherine Koss from the SEARCH study offered another reminder that social networks play a key role in PrEP use. The study made a valuable investment in looking at both community, social and structural aspects impacting health behaviors. SEARCH used an elaborate system of contact mapping and concluded that people who knew someone using PrEP were 57 percent more likely to use PrEP themselves. Destigmatizing and normalizing PrEP so that people talk about it when they choose to use it (or stop or restart it), and incorporating peer-to-peer support for all forms of PrEP into programs will likely be key.
Resistance happens among people taking CAB-LA but not necessarily during the “tail”. Long-acting products like CAB-LA include a period, after a person has stopped receiving injections, when the drug is still in the body but at levels that don’t provide protection against infection—often referred to as the “tail”. (For any PrEP strategy, there’s a blood-drug level that’s associated with prevention; you don’t need “some” PrEP drug but “enough” to reduce HIV risk.) There’s been real concern about drug resistance arising in people who acquire HIV during the tail. But in the three people who fell into this category in HPTN 083, none had HIV with genetic mutations that render it resistant to integrase inhibitors, the class of drug that includes cabotegravir. This number is too small to support conclusions about the risk of resistance, but in this handful of people, resistance didn’t emerge during the tail. When integrase inhibitor resistance did emerge in HPTN 083 participants, it was in people who were infected and also receiving the injections: one of the people who had HIV at the time of enrollment developed resistance, as did two people who acquired HIV during the oral lead-in phase (a trial period where individuals took the pill form of CAB-LA before receiving the injection, to ensure that the drug was well-tolerated). Two people who acquired HIV while receiving CAB-LA injections also developed integrase inhibitor resistance; two others had so little virus in their blood that the team could not get a resistance test. The take-home message—at least from this trial—is that the risk of resistance is greater when people acquire HIV while taking CAB-LA as prescribed than it is in people who’ve stopped receiving the injection, i.e., in the context of the “tail”. Cabotegravir is an integrase inhibitor, as is dolutegravir (DTG), which is used as first-line treatment in many settings. The people with integrase-inhibitor-resistant virus responded to alternative regimens, including TDF/FTC-efavirenz-based therapy and a regimen using a boosted protease inhibitor. In resource-constrained settings where countries have fully embraced DTG-based first-line therapy, it will be essential to plan for CAB-LA introduction in the context of treatment options for those with integrase inhibitor-resistance.
3. Injectable PrEP is “superior” to oral PrEP among people who can’t or don’t adhere to the daily pill. Landovitz also presented an analysis of serum and dried blood spot samples from HPTN 083 participants who were assigned to receive daily oral TDF/FTC, the comparison arm of the study. (In each arm, people also received a dummy, or “inert” version of the other product—people randomized to TDF/FTC received saline injections, people receiving CAB-LA injections received dummy pills.) Thirty-seven of the 39 individuals who were prescribed and counseled on the active daily pill and went on to acquire HIV had “suboptimal or non-adherent” levels of the drug in their samples at the time of infection. The study concluded that CAB-LA was “superior” to oral TDF/FTC. These new data clarify that people who received CAB-LA injections on schedule every two months had a substantially lower risk of HIV than people who received daily oral PrEP but did not or were not able to take it as prescribed. In other words, the difference in incidence rates relates to product use, and not the product itself.
One strategy is not inherently superior to the other, i.e., injections and daily oral PrEP each taken exactly as prescribed may well provide comparable levels of protection; the study didn’t evaluate this question.
Products exist in the real world, where people’s lives, circumstances, bodies and preferences play a role in what’s possible and pleasurable. In the real world—as several CROI presentations highlighted—starting and staying on daily oral PrEP is a challenge for some. The 083 data say much the same. “Choice is the answer,” declared the inimitable Dr. Linda-Gail Bekker in her plenary presentation, emphasizing that many oral PrEP programs see people using the strategy with complex patterns of starting and re-initiation. Understanding these patterns of use—and their impact on both individual risk and population incidence—is key to understanding the impact of PrEP.
Time to calculate the cost of progress—and of inaction
CROI also brought updates from product developers working on other types of long-acting PrEP, including a one-year implant containing the drug islatravir that could be placed under the skin for continuous protection, and a Dapivirine Vaginal Ring (DVR) that could be worn for three months. This is three times as long as a monthly ring that has received a favorable opinion from the European Medicines Agency and been recommended by the WHO. The International Partnership for Microbicides is presently submitting the monthly DVR for regulatory approval in a range of countries.
Islatravir (both as an annual implant and as a monthly pill, which is now in efficacy trials), a three-month ring and the six-monthly injectable lenacapavir look promising and would make choice-based programming real by expanding options and allowing prevention programs to meet people where they are. Long-acting PrEP might, for example, play a role in HIV prevention for postpartum or breastfeeding women. A study of PrEP use among pregnant and breastfeeding women found higher PrEP uptake among pregnant women compared to those who were not pregnant, and a drop-off in PrEP use during breastfeeding. But risk remains high for HIV-negative women who are breastfeeding—a reminder that matching prevention offerings with lifecycle events is a life-saving imperative.
All of these offerings will come at a cost, of course. A “superior” strategy like CAB-LA could reduce HIV in many people, but could also require new testing tools and counseling strategies—and still won’t work for everyone, including transgender individuals with buttock implants, unless an alternate injection site can be identified. A 90-day ring and a one-year implant will, similarly, offer transformative options for reducing HIV risk, but will not address rates of STIs that remain very high in many study populations. Transformative PrEP programs will be ones that offer choice in both HIV and contraceptive options, and sexual and reproductive health care, including STI prevention and treatment, for people of all gender identities and sexual orientations. It’s not possible to plan or budget for such programs without knowing the cost of the products themselves. To date, ViiV, the developer of CAB-LA, has not set a price for CAB-LA. A modeling study presented by Dr. Anne Neilan asked the question, “How much should we be willing to pay for CAB-LA for MSM and transgender women, compared to generic daily oral TDF/FTC, or branded FTC/TAF.” Using a set of prices specific to the Global North, Neilan and her colleagues proposed that the injection would need to be priced comparably to generic daily oral PrEP for it to be considered “cost effective”.
While such models and projections always have limitations, they provoke useful and necessary discussions. Will the price be set according to this calculation? If it is not, will gaps in PrEP access open as they once did with antiretrovirals for HIV, and as they have for COVID-19 vaccines? Or will the HIV field, in all its diversity, set an example for how to learn from history and advance equity for the future?
At the end of CROI, I was no more certain of this than when I might see my Ugandan friend again—or when Hassan and Gonsalves might next sit side by side in real life. But I did know, in my bones, that if there was progress towards equity and justice it would come from the human connections between activists, allies and friends that have always transcended physical distance out of love and anger, and a commitment to lasting change.
New Resources on AVAC.org!
Read on for a roundup of new resources on AVAC.org and PrEPWatch.org!
It’s one year into the COVID-19 pandemic, and the pace of advocacy for many people has only accelerated. In this time, when vast quantities of information—good and bad—must be sorted and priorities set, AVAC has been generating resources to help you keep up-to-date and to inform your advocacy.
HIVR4P in 3D
Okay, maybe it’s not really three dimensions; it’s more! Catch up on many angles of R4P starting with AVAC’s coverage:
- Making Do With “Good Enough”: A roundup of the first week of HIVR4P
- Prevention Unmasked: A roundup from week two
Recordings from the Advocates’ Corner went deep into the content and include insightful, lively exchanges:
- Understanding Broadly Neutralizing Antibodies
- COVID-19 & Vaccine Hesitancy
- Tracking Global PrEP Use and Trends
- A Conversation About Multipurpose Prevention
- What Does the CAB-LA Tail Mean for Implementation?
- Research Ethics—New Guidelines, Same Urgency
- A Review of the Pregnancy and Breastfeeding Women’s HIV Prevention Pipeline
- A Look at the 2021 Resource Tracking Report
- A Conversation About the Dapivirine Ring: What do the WHO Guidelines Mean for Access?
- Working with the Global Infrastructure: A Conversation with the COVID-19 Advocates Advisory Board
Funding for HIV Prevention R&D
The 16th annual analysis of funding trends in HIV Prevention R&D is out with 2019 data, and it can all be found on a newly launched website: www.hivresourcetracking.org. The annual report is a collaboration among AVAC, IAVI and UNAIDS, and the website includes 20 years of funding trends.
1 Million PrEP Initiations…almost
These two resources will help you understand the trends in PrEP uptake, and keep you up-to-date on PrEP initiations around the world, which is fast approaching 1 million but still well below global targets.
- AVAC’s PrEP Tracker (Updated December 2020)
- The Evolution of Oral PrEP Access: Tracking trends in global oral PrEP use over time (presented at HIVR4P)
Ending TB
AVAC, Partners in Health, Friends of the Global Fight, Results, Treatment Action Group (TAG) and the Zero TB Initiative collaborated on a report and a new website that includes case studies from around the world. Find out what successful programs in Cambodia, Ethiopia, Pakistan, Russia, South Africa and the United States have in common and learn more about the critical components needed to achieve the eradication of TB.
- How Can We End The Tuberculosis Epidemic?
- This report builds on the Translating Progress into Success to End the AIDS Epidemic report from AVAC, Friends and amfAR.
Protecting Global Gains
Two new vignettes on Protecting Global Gains explore how communities are adapting to maintain critical healthcare as COVID-19 threatens hard-won gains in global health. This project is a collaboration including AVAC, Partners in Health, Friends of the Global Fight, Results, Treatment Action Group (TAG) and the Zero TB Initiative.
The Evolution of Oral PrEP Access
A presentation on global trends in PrEP uptake since 2016, prepared for R4P. See breakdowns by country and analysis of lessons learned and continued use.
HIVR4P Virtual 2021—Prevention Unmasked: A roundup from week two
Last Friday marked the end of HIVR4P Virtual, and as AVAC reported last week, there are a number of excellent sources of news to catch up on all that was shared at the conference including coverage from aidsmap and Bhekisisa. In addition, AVAC roundups are available on our special HIV R4P page where you’ll also find recordings from the many great sessions hosted at the Advocates’ Corner, covering topics like vaccine hesitancy, research ethics, “the tail” in CAB-LA research and more. Consider checking out the Advocates’ Corner sessions you missed and continue the conversation on Engage!
Read on for a take on some of the key themes from the second week.
Prevention Unmasked: The second week of HIVR4P
by Emily Bass
Contents:
- The mucosa remain mysterious and critical to understanding early events in HIV
- Community-based research and activism: “Inside out” and “Outside in”
- Masks and partial, practical prevention
- PrEP unmasks longstanding questions about sexual pleasure and prevention of STIs
- Masks and the current pandemic moment
- Masked infections: A new mystery from the AMP trial
Masks are omnipresent these days: a public health tool, a commodity that’s a given for some, a luxury for others and—in politicized environments like in the United States—a symbol of one’s relationship to science and even vaccines. Masks were on my mind as I attended the second week of HIVR4P. What’s on the surface, what’s underneath? These were the questions that recurred, or seemed to, throughout the second week. Here are some ways the complexities and challenges in the field today appeared to me.
The mucosa remain mysterious and critical to understanding early events in HIV
For all the decades of research on HIV, with increasingly sophisticated techniques, assays and approaches, there are enduring unanswered questions—especially about the earliest events following the acquisition of HIV. In the context of sexual transmission, these events occur at the mucosa—the lining of the vagina, rectum and penis. A presentation involving long-term follow-up of participants in the Thai vaccine trial known as RV144 provided insights into when and how vaccine “boosts” deliver immune responses at mucosal tissue—a site that wasn’t sampled in the original trial. Alexandra Schuetz reported the finding that participants who received the boost at 12 and 15 or 18 months after their last shot had stronger immune responses, including at mucosal surfaces, than those who received it at 6 months. The timing of follow-up shots, and their impact on the types and location of immune responses is another area where there’s still a lot to learn—in HIV, COVID and other research arenas. Other research described below homed in on how STIs harm, and lactobacillus helps, mucosal barriers that block HIV infection, and explored the possibility that infections might start and remain contained for some time in this region.
Community-based research and activism: “Inside out” and “Outside in”
There’s a tendency in many fields to draw a distinction between “researchers” and “community”—one that many AVAC partners help to disrupt and redefine. One invaluable contribution to this effort came in an R4P presentation on the place of Black researchers in French and American institutions, which are shaped by white supremacy. Tashuna Albritton presented findings from the ETOILE study (Experiences of Tensions in Organizations and Interventions Leveraged for Empowerment and Prevention), a Franco-American study evaluating the community of researchers. The presentation featured the perspective of one Black PhD candidate who said, “You can have the same degree and yet what you are contributing to the conversation is not considered as legitimate.” ETOILE yielded a plainspoken reality: en Francais or in English, Black researchers are given the most labor-intensive and time-consuming tasks, and work in white-dominated power structures (while R4P works diligently to reflect the diversity of the field in its panels and program, there were instances of white-dominated panels at this conference, too). In these hierarchies, white senior researchers select white collaborators more often than Black ones. The mere fact that Albritton presented this work at HIV R4P helped unmask racism within the organizations where research is designed and conducted. The next conference will be a chance to take stock of how the organizers absorbed the messages.
Challenging traditional structures and distribution of power was also central to talks by Clever Chilende (TALC, Zambia) and Jacque Wambui (NEPHAK/independent activist, Kenya), who described two successful civil society efforts to challenge and change the way that research teams engage with community.
The PopART trial, also known as HPTN 071, was a “treatment as prevention” trial that measured HIV incidence in communities in Zambia and South Africa. Participants were randomized to one of three trial arms—Arm A received a combination prevention intervention with universal ART initiation on diagnosis; Arm B included the prevention intervention with ART according to local treatment guidelines; and Arm C was standard care. Researchers compared the rate of HIV incidence in trial communities to incidence in communities where ART was provided according to national guidelines. PopART started before universal test and treat became standard; midway through the trial national guidelines changed, the trial design shifted and ART became available on-demand to people in both Arms A and B. In Zambia, Chilende and his colleagues pressed PopART investigators to engage people living with HIV (PLHIV) as activists, advocates and watchdogs. They pursued an “outside-in” approach, whereby civil society advocates worked with the trial team and study communities to create forums for input, research literacy and independent monitoring. “The outside-in approach has the potential to improve transparency and accountability at a range of stakeholder levels,” Chilende concluded.
Jacque Wambui went beyond the role of trial communities, in her presentation on civil society engagement related to the ECHO trial, which evaluated the impact of three contraceptive methods on HIV risk. Wambui described how a Global Community Advisory Group, or GCAG, was convened by the ECHO trial team. Comprised of women from the trial countries and other geographies, the GCAG worked in tandem with an independent advocacy working group that brought together advocates from a range of countries and disciplines to share information and build consensus on key issues related to contraception, HIV risk and choice-based programming. The interplay between the GCAG and the advocacy working group (jointly convened by AVAC and ICW-EA) helped build civil society power. On the GCAG, a small number of individuals consulted directly with the trial team, ensuring a wide array of voices contributed to the conversation. (In the interest of full disclosure, I was part of the GCAG and helped co-convene the advisory group with ICW-EA. I cheered on Wambui as I watched from my tiny home office, pumping my fist and shouting “Womandla!”)
Masks and partial, practical prevention
In my neighborhood, most of us wear masks, but in other parts of New York City and in many parts of the US, many people don’t. Now that COVID-19 vaccines have arrived for some people in this country—even as the epidemic sky rockets in many other countries—Americans are in the privileged, if delusional, position of having conversations about when things will go back to “normal”. In other words, they want to know when they can take off their masks. If the HIV field has offered clarity on anything, it’s that there’s no single solution to a global pandemic. No new technology is ever going to supplant the need for action on multiple fronts, whether it’s safeguarding the human rights of key populations—as Johns Hopkins professor Chris Beyrer so eloquently described in his talk—or offering a range of options, which as Ram Prasad of Final Mile said in an insight-filled talk about the prevention needs and perspectives of South African adolescent girls and young women, include not just condoms or PrEP or any biomedical option, but “trust,” “partner loyalty,” and, yes, ‘periods of abstention.”
No single technology will supplant an array of approaches and, as presenters described, new options will need to be tested in a world where there is a larger array of HIV prevention strategies than at any point in the history of the epidemic. In a satellite session on “next generation” trial design convened by AVAC and partners, advocates and researchers described what trials might look like in a world where all the options are on the table. Mike Robertson from Merck described the design of a pair of trials known as IMPOWER-22 and IMPOWER-24 that will test a once-monthly oral ARV called islatravir for PrEP. In both trials, participants will receive both a monthly pill and a daily pill; in one arm of each trial, the monthly pill will be the active drug islatravir and the daily pill will be the placebo, while in the other, participants will receive active daily tenofovir-based PrEP and a placebo monthly pill in place of islatravir. This double-dummy, double-blind design means that participants will not know whether they received islatravir or tenofovir-based PrEP.
Because oral PrEP reduces incidence when taken correctly and consistently, and monthly islatravir may as well, rates of new HIV diagnoses could be very low in both arms of the trial. As Robertson described, Merck is planning to use recency assays—which detect very early HIV infection—in the communities where the trials take place to estimate what incidence would be among participants, if they were not in the trial. Robertson said the recency assays drawn from the community is “the best way to estimate incidence,” since incidence is expected to be extremely low in the trials.
In the same session, Moupali Das of Gilead Sciences presented their plans for two efficacy trials of their six-monthly injectable Lenacapavir that will also rely on recency assays to estimate background incidence in the same communities and populations where the trial will be carried out. Participants who remain HIV negative at the end of the initial phase will be offered the chance to be randomized into one of the trial arms. One of these trials will be conducted among cisgender women to study both injectable Lenacapavir and oral T/TAF as PrEP. The second is studying Lenacapavir among men who have sex with men, transgender women and gender non-binary individuals.
Recency assays have implications for people who test positive—it may pinpoint from whom someone acquired HIV. This raises a host of questions and could entail the need for critical supports including provider training, communications for communities and individuals and monitoring of potential harms to individuals receiving recency test results and to their partners—who might be identified as sources of HIV infection. Particular attention will need to be paid to use of recency testing in contexts where laws that criminalize HIV exposure or transmission are on the books. As AVAC and amfAR wrote in an issue brief on new HIV testing strategies and human rights concerns, including the use of recency testing, “potential harms that can come with such testing [include] a false sense of security and potential misuse as evidence of transmission.” The AVAC-convened Advocates’ Trial Design Academy and other allies raised these concerns in early consultations with Merck about the islatravir trials; in those discussions, the drug company said that it was open to suggestions and recommendations. With 2021 launch dates for these trials, the time is ripe for civil society engagement, which might include ensuring that all trials using recency assays have documentation on the legal environment regarding HIV criminalization in relevant communities; adequately-resourced, independent community oversight; and specific, proactive approaches to documenting adverse events such as gender-based or intimate-partner violence, disclosure or other outcomes that might emerge from use of recency testing.
In other words, these new trial designs aim to get answers about the range of products people want and need—but it will only work if “outside-in” advocacy is a reality. Its work that’s going to be critical and complex, and AVAC and partners including the Advocates’ Trial Design Academy are committed to doing this work. (The consultations related to the F/TAF trial design were also presented during the first week of R4P by my colleague Daisy Ouya.)
PrEP unmasks longstanding questions about sexual pleasure and prevention of STIs
One of the most delightful and, yes, downright pleasurable sessions of week two was a round of “speed debates” on key topics related to PrEP, pleasure and other sexually transmitted infections. In studies of communities where PrEP use is climbing, rates of HIV are dropping. In many of these same communities, rates of STIs like chlamydia, gonorrhea and syphilis are climbing—a trend that was underway prior to PrEP but that may also be impacted by changes in condom use as people rely on PrEP for HIV prevention and/or by the increasing rates of testing that come with PrEP programs. PrEP doesn’t protect people against those and other sexually transmitted infections, which has had some people arguing that a PrEP-plus-condom message was the best public health approach. Wear condoms and avoid STIs, Edwina Wright said, arguing (in character for the sake of debate) that most women don’t enjoy sex with another person anyway. Understand that PrEP makes sex pleasurable for people—and pleasure must be at the center of sexual health programming, said Mitzy Gafos, Alongside preventing and treating STIs, Gafos argued that research and programs must go beyond condoms to support peoples’ full range of needs and priorities.
The debate was high-spirited, the stakes serious: as Gladys Macharia described, in a cohort of East and Southern African men and women (part of IAVI’s protocol C) having an STI increased the risk of acquiring multiple strains of HIV, versus a single founder virus. Infection with multiple strains of HIV was associated with a faster CD4 cell decline. Having an STI, and treating it, can also disrupt the vaginal microbiome, as Eric Armstrong described in a close look at how, exactly, lactobacillus helps keep the vaginal microbiome healthy. While STIs disrupt the vaginal epithelium, a protective layer of tissue, lactobacillus helps keep it robust, at least in lab-based tissue cultures. Lactobacillus also recruited anti-inflammatory immune responses—the kinds of defenses that may help the body ward off HIV on its own. As people use more PrEP and, sometimes, fewer condoms, the message should be, “Here’s how to get pleasure and be safe.” The specifics of that remain to be seen.
A debate on whether to abandon the WHO-endorsed “syndromic management” approach to STIs saw an audience poll overwhelmingly in favor of moving to an approach that treats “etiologically”. Syndromic management involves offering treatment for the most likely infection based on symptoms, while an “etiological” approach refers to precise treatment for a confirmed infection e.g., chlamydia. Whether this shift will happen, what it will cost and what it will look like remains to be seen—but prevention and treatment of STIs must be a part of the expanded PrEP rollout that will happen as new strategies become available.
Masks and the current pandemic moment
Since the beginning of 2020, masks have gone well beyond metaphor. They are life-saving, essential protections as the world faces down the global COVID-19 pandemic. Scientists and advocates are striving toward a dual agenda for HIV and COVID-19 during key sessions at HIVR4P and at a one-day IAS COVID-19 Prevention Conference, open to R4P attendees. It wasn’t always easy. A session at the COVID-19 conference featuring GAVI leader Seth Berkley, activist Fatima Hassan from South Africa’s Health Justice Initiative and Peter Sands of the Global Fund to Fight AIDS, TB and Malaria got quickly to the heart of the matter: like AIDS, COVID-19 is a pandemic of global inequities, defined by grotesque imbalances in access to COVID-19 vaccines, lack of transparency on pricing, and intellectual property provisions that, until waived, favor patent-holding corporations over people.
In a satellite session at R4P, HIV researchers looked at what they could learn from COVID-19 and vice versa. Galit Alter from Harvard Medical School urged the field to let go of the “herd mentality,” and start thinking outside the box. Linda-Gail Bekker from the Desmond Tutu Health Foundation leapt in to say she’d be beating the herd from behind—urging greater speed, in line with the toll AIDS still plays worldwide.
Masked infections: A new mystery from the AMP trial
In the final session on the final full day of the conference, a frank and fascinating exchange among scientists, during a discussion on the antibody-mediated prevention (AMP) trial, explored the possibility of “masked HIV infections” among participants in AMP. Here, the term refers to an infection that occurred while a trial participant was receiving the active experimental product, but that was not diagnosed while the person was using the product. How might that happen? See the very first item in this write-up about the mysteries of the mucosa.
The very early events of HIV infection are still not well understood, but in the context of sexual exposure, HIV starts in the genital tract and then moves—often via immune cells—throughout the body. It’s long been hypothesized that a prevention strategy or even the body’s own defenses might be able to contain HIV in the genital tract, preventing it from establishing infection throughout the body. If a drug or antibody played a key role in that containment, would HIV infection progress when those agents are no longer present? In such a scenario, someone who previously tested HIV-negative would now test HIV-positive. “We have data in the higher-dose VRC01 group—more cases occurred in the tail of the study than in the placebo arm,” offered Peter Gilbert from Fred Hutchinson Cancer Research Center.
One reason this might have happened, session moderator Carl Dieffenbach of NIAID said: VRC01 might have had “a weak antiviral effect.” In other words, the bump in what looked like new infections at the end, or tail, of the study in people who received VRC01 might have actually been the appearance, in the bloodstream of HIV that VRC01 had previously contained in a localized area, remaining undetected until VRC01 had washed out of the body. In intriguing new-to-me remarks, panelist Mike Cohen of the HPTN chimed in that masked infections might have happened in the CAB-LA trials, too. AVAC is following up to clarify and understand these hypotheses, but in that session, Cohen suggested that “masked infections” may be a reality in long-acting prevention. “That phenomenon almost certainly exists,” said Cohen, who went on to say that CAB-LA trials had 15 men versus and four women in the CAB-LA trials diagnosed with what he described as “a not-easily detectable infection.” Hearing investigators think aloud about what they’ve seen in trials—particularly what’s perplexing them—can be enormously revealing. In this case, there’s clearly far more to understand and learn.
Advocates have experience with the possibility of “false positive” HIV tests—especially in the context of HIV vaccine trials where the vaccine induces antibodies that cause a positive result on HIV-antibody tests. But a “false negative” is new terrain. What does the possibility of a false negative mean for informed consent processes, post-trial follow-up, the potential need for more sensitive HIV tests in the context of HIV programs, public health messaging, and community understanding of product efficacy? These critical questions began buzzing as soon as the session ended in an advocates’ debrief, and will be hashed out for months to come.
The AMP session also left open questions about how to use and describe the assay devised to study cases of HIV acquired among participants in the AMP study. That assay, known as the TZM-bl neutralization assay, involved sequencing the genes of viruses from participants, and then isolating genetic sequence encoding the envelope protein, known as “env”. The env sequences from participants were then used to form a new viral particle known as a “pseudotype”—which contains the isolated env sequence along with standardized, lab-adapted viral proteins. Making pseudotypic particles—in which the only difference is the env sequence—allows researchers to make controlled comparisons. In this case, the primary question was how the pseudotypes viruses from AMP participants responded to VRC01—whether the antibody neutralized it and, if so, at what concentration. The AMP investigators say that the trials validated the TMZ-bl assay as a means of predicting the breadth and potency of future bNAbs, or bNAb combinations, against a range of viruses. This predictive ability could be used to select bNAbs alone or in combination for future studies. Not so fast, said panelist Michel Nussenzweig, from the Rockefeller University who said that the field had to see AMP as “a disappointment,” and warned that for some bNAbs in development their ability to neutralize viruses was markedly different, depending on whether they were tested against pseudotypes or whole viruses, isolated and cloned from human samples. The investigators jousted back and forth about the relevance of the assay—settling for “both/and”—TZM-bl isn’t a total solution, but it’s an advance that moves the field a step in the right direction, if not across the finish line.
And that, in a way, is the best one can ask for right now—steps in the right direction, a realistic expectation of what a “finish line” might look like, and the understanding that we might still be wearing masks—real and metaphorical—on the other side. That’s especially true if the work doesn’t align with principles of justice and equity. In her talk last Wednesday, Dazon Dixon Diallo offered a quote from Paolo Freire, the anthropologist and theorist of inequality, that is as succinct and powerful a summation of this reality as any I know. And at the end of this roundup, of this most unusual conference in this most perilous time, Freire, via Dixon Diallo, has the last word: “Attempting to liberate the oppressed without their reflective participation in the act of liberation, is to treat them as objects to be saved from a burning building.”
HIVR4P Virtual 2021—Making Do With “Good Enough”: A roundup of the first week of HIVR4P
There are a number of excellent sources of news on the conference including coverage from aidsmap, Bhekisisa and this roundup from AVAC. Last week, we also highlighted findings from the AMP study of passive immunization with an antibody known as VRC01, which did not show overall protection in two studies, but did prevent infection by HIV that was highly-sensitive to the antibody. AVAC is finalizing an Advocates’ Guide to the AMP Results and hosted a lively discussion of the findings in the Advocates’ Corner—a vibrant virtual space that’s open throughout the conference.
HIV isn’t the only field looking at antibodies for treatment and prevention. On Tuesday, a one-day conference on COVID-19 prevention will look at the science and politics of developing new vaccines and therapeutics, and the work to be done to close the yawning gap in access to approved COVID-19 vaccines. You can follow along on social media #COVIDconf and look for AVAC to recap highlights in our week 2 R4P round-up next week.
As always, check out AVAC’s special webpage for the latest on all things R4P and IAS COVID-19 Prevention Conference.
Below is a longer take on some of the key themes from the first week.
Making Do With “Good Enough”: The first week of HIVR4P
by Emily Bass
The first week of the HIV Research for Prevention (HIVR4P) Conference wrapped up with a surprising and welcome sense of intimacy and embeddedness in the real world. Presenters spoke from their homes and offices, with bookshelves, art, the occasional guitar. Instead of speaking from podiums in windowless conference rooms with dimmed lights, speakers spoke from wherever they were—with the light slanting often evoking a time zone far away from where another viewer was sitting. Moments of silence for those lost to COVID-19 often started and ended with the session chair breathing deep. During a moving tribute to Gita Ramjee, lost to COVID-19 in the earliest days of the epidemic, I was alone in my apartment, and also together with the whole conference grieving. We were not together but we did the best we could.
In many ways, the experience of being “at” the conference held the core lessons for the field from the presentations: Meet people where they are; do not make the perfect the enemy of the good; listen to each other, even when it’s hard.
Meet people where they are
A range of evidence from studies of oral PrEP programs underscored the importance of creating programs that meet people’s needs. Kenya’s national PrEP program was, initially, “convenient to the system, not to the user,” said Daniel Were of Jhpiego, a partner in the Kenyan Jilinde program for PrEP rollout. “Simplifying, demedicalizing and decentralizing,” PrEP in Thailand, including shifting delivery into key population-led service sites helped the Thai PrEP program achieve a 300 percent increase in uptake among transgender people, reported Nittaya Phanuphakat the same session. In South Africa, a study of oral PrEP among pregnant women—which also found higher initiation and continuation among these women compared to women who were not pregnant—noticed a major dropoff in refills after COVID-19. The research team got on the phone and asked women what they needed, and acted on what they heard. To alleviate fears of acquiring COVID-19 in long clinic queues, the program offered PrEP pickup at clinic gates. Women just had to text and someone brought the meds they needed. The SEARCH study, which documented incidence declines after PrEP introduction, offered refills on beaches, at home and in informal, non-clinic based settings. “Any slight inconvenience and most [people who use PrEP] are likely to drop along the way,” Were said. “I might go this month and not go next month, because my finances might not be able to carry me,” said Josephine Aseme, a Nigerian health activist and current AVAC Fellow who also uses PrEP.
Implementers of and advocates for oral PrEP programs leaned into the work of designing programs that help people start PrEP not just once but several times. Data from a range of studies show that people start, stop and restart oral PrEP; in SEARCH and Jilinde, this cycling was lower among people who remained at high risk of HIV. Cycling on and off an antiretroviral for prevention runs counter to the antiretroviral treatment model, in which people who start ART are generally asked to remain on treatment for life. But, as AVAC Report discussed in 2019, measuring performance against PrEP initiation and retention may not give a clear sense of PrEP impact in the community. Better measures of “effective use” wouldn’t just look at whether someone stopped or started but at how that pattern related to their own risk; measures of impact might look at coverage within a community as measured by refills or volume of drug dispensed over a certain period of time. Nittaya Phanuphak said that Thai policy makers were asking “traditional questions” about retention. In response, the Thai PrEP implementers are working to familiarize their government counterparts with the notion of “effective use.”
Do not make perfect the enemy of the good
The dichotomy between “traditional” measures of retention and newer approaches to measuring effective use of oral PrEP played out in the conference itself; in some sessions, low retention rates in oral PrEP program were called “sobering” or used to make the case for emerging prevention strategies like the Dapivirine Vaginal Ring (DVR), which was recommended by the World Health Organization as part of combination HIV prevention the day before the conference began, or long-acting injectable cabotegravir (CAB-LA).
CAB-LA made waves at the meeting, with new and expanded data from the HPTN 084 trial in cisgender women. In late 2019, the trial announced initial efficacy findings following an interim DSMB review; the data have not yet been published, but data presented by Sinead Delaney-Moretlwe showed that incidence was low in women randomized to receive both oral and injectable PrEP. In women assigned to the daily pill, incidence was less than 2 percent; in those assigned to receive the injectable it was less than one percent. By comparison, incidence in other HIV prevention trials in similar populations over the past 15 years have consistently been closer to 4 percent. Both options were safe and reduced risk. The injectable was comparatively more effective—reducing risk by 89 percent more compared to women taking oral PrEP. There were equivalent rates of adverse events reported in both trial arms, and data from women who became pregnant during the course of the trial showed no safety issues related to product use. Participants who became pregnant were offered open-label TDF/FTC but all injections were discontinued. HPTN 084 has little data on the so-called “tail”, the period in which cabotegravir is still in the blood but not at levels that would prevent infection. A person who stopped injections but remained at risk of HIV would need to use another prevention option to reduce risk, but little is known about how long people would need to be concerned about the tail. Delaney-Moretlwe said that there isn’t sufficient data from 084—where very few participants discontinued the injection—and that this information could come from open-label extension trials.
The conference also brought data, presented by Sharon Hillier, from a Phase IIa trial of a monthly PrEP pill called Islatravir. The data are from 192 participants and found that the monthly dose was safe and well tolerated, and that it led to blood levels well above the protective threshold that the investigators had calculated based on animal data and studies of Islatravir as a therapeutic treatment in people living with HIV.
In discussions of the monthly pill and the bimonthly injection, speakers celebrated the coming moment when PrEP-focused programs will have a range of options—from a vaginal ring, to a daily or monthly pill, to an injection. Such programs would have much in common with the ideal contraceptive service, which offers people a range of options. At times, the conversation seemed to veer away from the reality of these contraceptive programs—which, in many countries in East and Southern Africa, offer a limited number of choices or, since COVID-19, no options at all during periods of stockout, service disruption or lockdown. Several speakers said that women in sub-Saharan Africa “like” or “prefer” injectable hormonal contraceptives, and while this is true for some women, it is also true that many women receive the injectable because it is what’s offered to them—or because it is the only long-acting discrete method on the shelf.
This week at R4P, Jacque Wambui, an HIV prevention and women’s health activist from Kenya, will present civil society work focused on precisely this issue in an abstract on civil society advocacy related to the ECHO trial. Moving forward, it’s going to be essential for prevention advocates of all affiliations—researchers, activists, potential users of products, policy makers and funders—to look at the reality of “choice” in the context of constrained options, and ensure that biomedically-focused PrEP programs offer options preferred by people, not just the health system.
Listen to each other—even when it’s hard
A reminder of the reason why oral PrEP is critical right now—and for the foreseeable future—came from Gcobisa Madlolo, a South African feminist activist who talked frankly about taking oral PrEP to be “rape ready”, her own experiences with sexual violence and the ways that she and her friends support each other in strategies, including PrEP use, that provide resilience and safety in the face of daily threats to women’s bodily autonomy. Conversations about people discontinuing injectables, or other methods, because they’re no longer “at risk” are important—so is understanding that in every place, there are some people who cannot choose if, when and how they are at risk of HIV, sexual and other forms of violence. The number of people who do not have that fundamental choice has risen since the onset of COVID-19 and is the devastating new context in which HIV prevention must function.
More tough, necessary conversations will occur in the context of the new UNAIDS Ethical considerations in HIV prevention trials guidance—a major update to a document that has long served as an essential framework for trial design and conduct. This is the first update in 13 years. When the last version was published in combination with the Good Participatory Practice guidelines, the field was grappling with whether participants in prevention trials should be offered the best proven standard of prevention, even if a given strategy such as voluntary medical male circumcision or oral PrEP was not available in the country or community. The alternative view held that trials needed to offer the best available standard—tying prevention to national public health programming. Guidance Point 11 of the new ethical considerations states that participants should be offered the WHO-recommended package of interventions at every stage of the trial—including pre-enrollment—and to cohort participants. This is a major shift with immediate implications for trials like the upcoming Phase III study of Islatravir—with WHO recommendation of the Dapivirine Vaginal Ring, the decision not to offer it requires, per the guidance, consultation and discussion. That’s just one example of a complex issue that the new guidance will help to shape conversations on. In the coming months, AVAC and partners, working with trial teams on a range of research projects, will ensure that ongoing conversations and decisions reflect the new reality.
More substantive dialogue is coming this week and I, for one, can’t wait. This year’s conference, it’s real-time questions and comments during official sessions and virtual “hallway” discussions in the Advocates’ Corner, is a reminder of how HIV activists and advocates of all stripes have always found ways to be connected, build community and share strength, even in the hardest of times.
See you in the virtual hallways this week!