Yvette is currently working at the Centre for Communication Impact (formerly JHHESA). She is a founding member of the new Advocacy for Prevention of HIV and AIDS (APHA) in South Africa, a former AVAC Advocacy Fellow and a leader in the country’s HIV prevention movement for young women. This blog is one in a series written by community scholars who attended CROI 2016.
My first CROI and it was not the science that was overwhelming…
When I was presented with the opportunity to apply for a scholarship to attend this very scientific meeting and conference as a community educator I was thrilled and most of all exited that I would be in the presence of all the scientists who’s work I have torn to shreds to get my communities in Mpumalanga, Limpopo and KwaZulu-Natal (KZN) to understand. As a community educator I have had to explain microbicides, PrEP, TasP, HIV vaccines and the BIG one: why we still don’t have a cure. Also, I have had to explain why it is important that we know the new research and why a lot of the HIV research happens in South Africa.
Upon my arrival I was overwhelmed by the beauty of the city; the buildings all looked old but they were a beautiful sight. When I left South Africa, the university students were burning old art and statues because it reminded them of our painful past. As I was driving from the airport upon my arrival in Boston, I saw a few homemade banners of Black Lives Matter in the city attached to these old buildings. They were not torn apart; they looked just like they belonged there. They were not threatening each other—the old building and the new feelings of we matter, black lives matter. I wish I had stopped my driver to take a picture.
I was invited to a pre-conference on cure research, on where we are. Because of my suspicions with cure and quacks, I thought I would only last 30 minutes at the most. How wrong I was. I was intrigued by how much research is happening and the amount of work that researchers were putting in trying to unlock this mystery. I was overwhelmed about how much of this research is happening in South Africa and that one study was actually happening in KZN. Research showed that even with focused interventions of counselling and empowerment skills, there were no differences in the new infection rates of young women in the program and those not in the program. Suffice it to say I was there the whole day and did not want to miss a presentation let alone a slide.
The next day was the first day of the conference and our day started at 7 am. I was jet lagged but did not want to miss anything. Not even the cold could keep me in bed. For the duration of the conference we had an opportunity to interact with the scientists, thanks to a great initiative by the BAI, AVAC and CROI Community Liaison Subcommittee. These morning meetings were a space where we as community advocates could come together and learn from each other. I realised that I knew about 30 percent of the community through our work and most of all our very vocal online presence on Facebook. I did not want to show my anxiety around the pending Ring and ASPIRE results so I would walk around meditating that it works. After all, my girls were hoping it does. The day the results were going to be announced I dressed like a winner—I wanted women to win.
When Dr Annalene Nel, lead researcher on the <Ring Study, mentioned a “significant” efficacy result, I did not know what to feel. I was overwhelmed by the word and I knew it worked. I knew our work was cut out for us as advocates. A lot of questions still remained unanswered for the young women but at least the ring works and we know that now. I was happy, very happy. I immediately announced it on my FB Page and I received so many inboxes from young women— both positive and negative—and most told me there is HOPE. Seeing Annalene made me very emotional because unlike the other researchers at CROI she did not see “subjects”— a word that made be shiver every time it was mentioned at sessions throughout the conference referring to research participants. She saw trial participants as young women, some of whom she had met throughout out the study period. I knew she was counting on support and I walked up to her and I told her what this means to the young women I worked with. Annalene received a standing ovation. I was happy—finally a woman for other women. For a feminist like me it mattered that Dr. Annalene was at the forefront. It mattered that she was OK. I was overwhelmed with pride to be South African at this conference and it mostly mattered that I was a woman at the conference too. The rectal microbicides trial results were also positive news for HIV prevention.
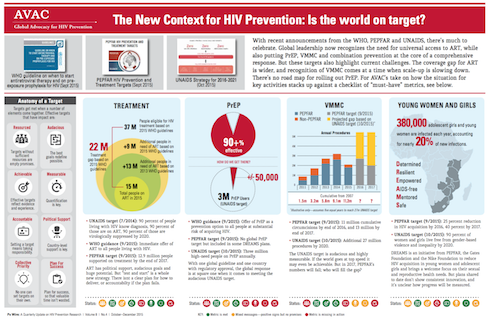
At this conference I learnt that there are more HIV prevention tools than there has ever been before. I was left wondering who these prevention tools are developed for. Why is it that if treatment is prevention are there not more visible TasP campaigns, despite UNAIDS’ 90-90-90 and MSF’s Getting to Undetectable? Why is it taking policy makers so long to implement early treatment when the benefits from the START study are so overwhelmingly positive? The benefits, especially for those living with HIV, are lowered risk of cancer, among other risks. This cannot be rocket science, especially seeing that women living with HIV are at such high risk of cervical cancer. Why is it that if being virally suppressed reduces the risk of passing the virus on to others, we are not including this in mass HIV communications? If PrEP works and can help defeat HIV, why are there only four countries that have approved the use of Truvada as PrEP? (Since CROI, two more countries have approved PrEP.) And why is South Africa not moving any faster with rolling out PrEP to those who need it?
I was also swayed in my initial stance against home testing, thinking pre- and post-testing might get lost in the process. However, if this is not the case and home testing will increase the number of men who test, I am now for it.
It was a week of late nights and early cold mornings, a week of appreciating the science. By including the community educators at CROI 2016 I hope the scientists will appreciate the work done by communities and most of all advocates. We will only appreciate your science if you appreciate our stories. And yes it does matter who delivers the news to whom: Male, Female Gay or Straight.
Thank you for the opportunity, the support and the guidance AVAC, BAI, CROI, Sister Love and all the other sponsors.