The NIH announced a new study to test three mRNA-based HIV vaccine candidates. This study follows an announcement in January from IAVI about another mRNA-based HIV vaccine study. This snapshot compares the two studies.
Experimental mRNA-based Preventive HIV Vaccine Phase 1 Trials
Essential Reading and Resources
AVAC and our partners have been busy the first couple of months of 2022. Here is a round-up of essential reading and new and updated resources including HIV treatment studies, PrEP, stakeholder engagement, research fundamentals and more.
Highlights From CROI
Our coverage of week one at CROI featured updates on data related to the Dapivirine Vaginal Ring, injectable cabotegravir (CAB) as PrEP, vaccine science and cure research. And our week two summary looked at symposia that explored how to reach key populations, the critical role of choice in effective HIV prevention, and the status of research on preventing sexually transmitted infections (STIs).
HIV Treatment Studies During COVID-19
A February commentary in JIAS, coauthored by AVACer Maureen Luba with a host of other experts on the topic, offered recommendations for the ethical continuation of treatment research among people living with HIV in the context of COVID-19: Mitigation strategies to safely conduct HIV treatment research in the context of COVID-19.
Transgender Voices: Call to action
The Lancet’s February publication included a public letter from leading voices in the transgender community working in HIV research and public health. Titled, Research on transgender people must benefit transgender people, it called out exploitation in academic research and “called in” researchers to pursue priorities that offer direct benefit to transgender communities and to rely on resources such as No Data No More: Manifesto to Align HIV Prevention Research with Trans and Gender Diverse Realities.
Stakeholder Engagement Must Overcome Tokenism and More
The ethics review process is a critical opportunity for stakeholder engagement. AVACer Jessica Salzwedel, CASPR partner Cathy Slack and other co-authors explore three themes that can make or break effective engagement in a new article in the Journal of Empirical Research on Human Research Ethics: “It’s Almost as if Stakeholder Engagement is the Annoying ‘Have-to-do’…”: Can Ethics Review Help Address the “3 Ts” of Tokenism, Toxicity, and Tailoring in Stakeholder Engagement?
South Africa Is Talking About Injectable CAB As PrEP and Getting Rollout Right
Leading South African publication on public interest health journalism, Spotlight, reported on the excitement among advocates – and the need to plan now – to add injectable CAB as PrEP to the HIV prevention options currently available. Check out two new stories: Start planning HIV prevention injection rollout, experts say and Prioritise HIV prevention injection, activists say.
Many Angles On PrEP And Resources For Advocacy
For context on injectable CAB as PrEP, resources to support the rollout of the ring, and a look at the research and development pipeline of next generation PrEP, check out PrEP and more PrEP: An update and important resources.
Research Fundamentals
What is an endpoint in clinical research and why does it matter? POZ magazine’s February issue featured a transcript of AVAC’s Px Pulse podcast on this question. Hear the original nine-minute podcast here. And you can find the Px Pulse archive here.
We hope these resources offer you the context and tools you need to use your passion and add your voice to the work ahead.
Research Fundamentals: What is an endpoint?

The next installment of our Px Pulse series Research Fundamentals, explaining key concepts in HIV prevention research, is up. In this episode, Px Pulse host Jeanne Baron and Matthew Rose, a veteran HIV advocate and now Director at Global Health Strategies, look at endpoints in research.
Endpoints are a crucial component in every clinical trial but they are not always well understood. In addition, advocates can and should play a role, reviewing endpoints and interrogating how well the trial will serve communities that need HIV prevention.
Joining us to explore all this are:
- Dave Glidden, Professor of Epidemiology & Bio-statistics at UC San Francisco
- Erica Lessem, Senior Strategist for NYC Dept. of Health and Mental Hygiene, former Deputy Executive Director, Treatment Action Group
- Meagan O’Brien, Senior Medical Director of Early Clinical Development & Clinical Experimental Sciences at Regeneron
Listen to learn how endpoints are used in clinical research, why they change overtime, and what matters most about endpoints for advocates and researchers alike.
New Report: HIV Prevention R&D funding drops again
Today, we and partners are proud to release the annual HIV Prevention Research and Development Investments Report, with important findings for our collective advocacy. The report reveals a growing mismatch between the current promise of HIV prevention R&D, and continuing declines in the funding available. This decline affects both funding for research on new interventions and funding to expand access to existing prevention tools. The new report is based on outreach to 215 funders of HIV prevention R&D in the public, philanthropic and commercial sectors and includes 2020 funding data.
The latest data shows funding for HIV prevention R&D dropped by US$54 million (4.4 percent) in 2020. This second consecutive annual decrease is part of an eight-year trend of flat or declining funding for HIV prevention R&D.
The report also finds that financial support for HIV prevention R&D is almost entirely dependent on public sector funders, notably from the United States, and on one key United States-based philanthropic funder, the Bill & Melinda Gates Foundation. Commercial sector funding, already extremely low, dropped again in this year’s survey.
“These concerning trends in funding come at a promising but very demanding moment in efforts to control the pandemic,” said Mitchell Warren, executive director of AVAC, which coordinates the Resource Working Group with the International AIDS Vaccine Initiative (IAVI) and the Joint United Nations Programme on HIV/AIDS (UNAIDS). “Funding is declining just as the field confronts a new generation of opportunities and challenges.”
This high stakes environment includes: new products readying for introduction, such as injectable cabotegravir for PrEP and the Dapivirine Vaginal Ring; international support for ambitious new global targets for ending the epidemic; initial proof of concept of antibody-based prevention; and urgently needed new thinking for HIV vaccine development as recent trials have experienced setbacks and new technologies such as mRNA succeed against COVID-19.
Key findings from the report include:
HIV prevention R&D is highly overdependent on a few key funders, and much of the world is not contributing at the levels seen in prior years:
- HIV prevention R&D funding relies almost exclusively on the public sector, particularly the US public sector. The trend toward an overdependence on a small number of large investors, which the Working Group has surfaced and cautioned against in the past, intensified further in 2020.
- Globally, the public sector accounts for 86 percent of prevention R&D funding, with 92 percent of that coming from the US public sector.
- European public sector investments represent only 7 percent of the global total. While European public sector investment increased by 57 percent in 2020, it is still barely half of the US$124 million the European public sector contributed in 2009.
- The entire rest of the world accounted for only US$14 million, or just 1 percent of total public sector funding.
- Philanthropic funding, consisting almost exclusively of funding from the Bill & Melinda Gates Foundation, declined 20 percent in 2020 to US$127 million or 12 percent of the total global investment.
- Reported commercial sector support for HIV prevention R&D, already the lowest segment of investment, fell by 55 percent to US$31 million, or just 3 percent of the total, in 2020. While total commercial investment may be underreported, trends over time from the data collected show commercial sector investments is, by far, the smallest piece of the funding pie for HIV prevention R&D.
Funding dropped in 2020 across a number of key segments, including:
Preventive vaccine R&D: With two large-scale HIV vaccine trials underway, and dozens of new approaches under investigation, funding for preventive HIV vaccine R&D decreased by 5.5 percent or US$46 million in 2020 to US$802 million. While different European countries have increased or decreased their investments, overall European public sector investment in HIV vaccine R&D decreased 31 percent in 2020, to US$48 million.
R&D for PrEP, including pills, implants, injections: While uptake of oral PrEP grew substantially in 2020, and multiple recent research studies have demonstrated the potential impact of a range of PrEP options including long-acting injections, pills and implants, global investment in PrEP R&D declined 2 percent in 2020 to US$107 million. While US public sector donors increased funding for PrEP R&D by 5 percent, and commercial sector investment increased by 21 percent to US$24 million, neither was enough to overcome a 42 percent decline in funding from the philanthropic sector.
Voluntary Medical Male Circumcision (VMMC): As a number of studies affirmed the efficacy of VMMC over a decade ago, funding in the field is focused on implementation science, behavioral studies and advocacy and policy, each of which is vital to extending the reach and impact of this highly effective prevention tool. Yet investment in VMMC decreased by 37 percent to just US$6 million in 2020, almost all of which came from a single donor, the Bill & Melinda Gates Foundation.
Preventing vertical transmission: Prevention of mother-to-child transmission of HIV (PMTCT) remains a key prevention priority, but funding for PMTCT R&D decreased by 29 percent in 2020, from US$35 million to US$25 million. The decline is attributed to the loss of the Bill & Melinda Gates Foundation from the list of PMTCT R&D funders, and to decreases in funding from public donors. US public sector funding for PMTCT R&D fell 22 percent to US$22 million in 2020. European funding also fell more than 60 percent, from US$3.4 million in 2019 to US$1.3 million in 2020.
Only two areas of prevention R&D funding showed small increases in funding, including:
Treatment as Prevention (TasP): Long neglected in HIV prevention investment, funding for treatment as prevention (TasP) R&D increased from $1.7million to US$9 million in 2020. The increase came from philanthropy, notably the Bill & Melinda Gates Foundation (US$5 million) and the Wellcome Trust (US$1 million).
While TasP R&D funding is small overall, this increase is a hopeful sign that TasP may once again receive its appropriate focus as priority for HIV prevention research.
Microbicides: After multiple years of decline, investment in microbicide R&D registered a very small increase (0.4 percent or US$0.6 million) to US$145 million in 2020. Concerningly, there is even less diversity in microbicide funding than in HIV prevention R&D overall, with the public sector providing 99 percent of microbicide R&D resources.
While this tiny increase is a hopeful sign, it does not match the scope of the promise of microbicides. One key product, the Dapivirine Vaginal Ring, is now recommended by the WHO as an additional HIV prevention option. In addition, a range of promising microbicide strategies are under investigation. One, a 90-day dual-purpose vaginal ring designed to confer both contraceptive and HIV protection, was found to be effective in early testing.
This is the 16th annual report from the Resource Tracking for HIV Prevention Research & Development Working Group. Go to HIVResourceTracking.org to explore the key findings, funding trends, and previous reports in depth and follow the conversation on Twitter #HIVResearchFunding.
Press Release
HIV Prevention R&D Funding Drops Again, Even as Major Scientific Advances Require Support
A Worrying Trend Toward Overreliance on a Few Funders Increased in 2020
Contact
Kay Marshall, +1 (347) 249-6375, [email protected]
December 8, 2021 – The annual HIV Prevention Research and Development Investments Report reveals a growing mismatch between the current promise of HIV prevention R&D, and consistent declines in the funding available to both research new HIV prevention approaches and expand access to the prevention tools available today. The 2020 report, based on outreach to 215 funders of HIV prevention R&D in the public, philanthropic and commercial sectors, is the 16th annual report from the Resource Tracking for HIV Prevention Research & Development Working Group.
According to this year’s report, funding for HIV prevention R&D dropped by US$54 million (4.4 percent) in 2020. This second consecutive annual decrease is part of an eight-year trend of flat or declining funding for HIV prevention R&D. The report also finds that financial support for HIV prevention R&D is almost entirely dependent on public sector funders, notably from the United States, and on one key United States-based philanthropic funder, the Bill & Melinda Gates Foundation. Commercial sector funding, already extremely low, dropped again in this year’s survey.
“These concerning trends in funding come at a promising but very demanding moment in efforts to control the pandemic,” said Mitchell Warren, executive director of AVAC, which coordinates the Resource Working Group with the International AIDS Vaccine Initiative (IAVI) and the Joint United Nations Programme on HIV/AIDS (UNAIDS). “Funding is declining just as the field confronts a new generation of opportunities and challenges. These include the introduction of injectable cabotegravir for PrEP and the Dapivirine Vaginal Ring, ambitious new global targets for ending the epidemic, initial proof of concept of antibody-based prevention, and the need to rethink HIV vaccine development in light of setbacks in recent trials and the possible promise of mRNA and other vaccine approaches.”
Among the key findings from the annual HIV Prevention Research and Development Investments Report are the following:
HIV prevention R&D is highly overdependent on a few key funders, and much of the world is not contributing at the levels seen in prior years.
- HIV prevention R&D funding relies almost exclusively on the public sector, particularly the US public sector. The trend toward an overdependence on a small number of large investors, which the Working Group has surfaced and cautioned against in the past, intensified further in 2020.
- Globally, the public sector accounts for 86 percent of prevention R&D funding, with 92 percent of that coming from the US public sector.
- European public sector investments represent only 7 percent of the global total. While European public sector investment increased by 57 percent in 2020, it is still barely half of the US$124 million the European public sector contributed in 2009.
- The entire rest of the world accounted for only US$14 million, or just 1 percent of total public sector funding.
- Philanthropic funding, consisting almost exclusively of funding from the Bill & Melinda Gates Foundation, declined 20 percent in 2020 to US$127 million or 12 percent of the total global investment.
- Reported commercial sector support for HIV prevention R&D, already the lowest segment of investment, fell by 55 percent to US$31 million, or just 3 percent of the total, in 2020. While total commercial investment may be underreported, it is still the smallest piece of the HIV prevention R&D funding pie.
Funding dropped in 2020 across a number of key HIV prevention R&D segments, including:
Preventive vaccine R&D: With two large-scale HIV vaccine trials underway, and dozens of new approaches under investigation, funding for preventive HIV vaccine R&D decreased by 5.5 percent or US$46 million in 2020 to US$802 million. While different European countries have increased or decreased their investments, overall European public sector investment in HIV vaccine R&D decreased 31 percent in 2020, to US$48 million.
R&D for PrEP, including pills, implants and injections: While uptake of oral PrEP grew substantially in 2020, and multiple recent research studies have demonstrated the potential impact of PrEP in the form of long-acting injections, pills, implants and rings, global investment in PrEP R&D declined 2 percent in 2020 to US$107 million. While US public sector donors increased funding for PrEP R&D by 5 percent, and commercial sector investment increased by 21 percent to US$24 million, neither was enough to overcome a 42 percent decline in funding from the philanthropic sector.
Voluntary Medical Male Circumcision (VMMC): As a number of studies affirmed the efficacy of VMMC over a decade ago, funding in the field is focused on implementation science, behavioral studies and advocacy and policy, each of which is vital to extending the reach and impact of this highly effective prevention tool. Yet investment in VMMC decreased by 37 percent to just US$6 million in 2020, almost all of which came from a single donor, the Bill & Melinda Gates Foundation.
Preventing vertical transmission: Prevention of mother-to-child transmission of HIV (PMTCT) remains a key prevention priority, but funding for PMTCT R&D decreased by 29 percent in 2020, from US$35 million to US$25 million. The decline is attributed to the loss of the Bill & Melinda Gates Foundation from the list of PMTCT R&D funders, and to decreases in funding from public donors. US public sector funding for PMTCT R&D fell 22 percent to US$22 million in 2020. European funding also fell more than 60 percent, from US$3.4 million in 2019 to US$1.3 million in 2020.
Only two areas of prevention R&D funding showed small increases in funding, including:
Treatment as Prevention (TasP): Long neglected in HIV prevention investment, funding for TasP R&D increased from $1.7million to US$9 million in 2020. The increase came from philanthropy, notably the Bill & Melinda Gates Foundation (US$5 million) and the Wellcome Trust (US$1 million).
While TasP R&D funding is small overall, this increase is a hopeful sign that TasP may once again receive its appropriate focus as priority for HIV prevention research.
Microbicides: After multiple years of decline, investment in microbicide R&D registered a very small increase (0.4 percent or US$ 0.6 million) to US$145 million in 2020. Concerningly, there is even less diversity in microbicide funding than in HIV prevention R&D overall, with the public sector providing 99 percent of microbicide R&D resources.
While this tiny increase is a hopeful sign, it does not match the scope of the promise of this approach. One key product, the Dapivirine Vaginal Ring, is now recommended by the WHO as an additional HIV prevention option. In addition, a range of promising microbicide strategies are under investigation. One, a 90-day dual-purpose vaginal ring designed to confer both contraceptive and HIV protection, was found to be effective in early testing.
Pandemic preparedness requires greater investment in HIV, other current health crises
“The response to the COVID-19 pandemic has demonstrated that when there’s political will, global solidarity, and significant financial investments, rapid developments of new prevention technologies such as vaccines happen,” said Shannon Hader, deputy executive director of programme, UNAIDS. “This is the time to mobilize investments in HIV service delivery/prevention research and galvanize momentum to achieve the broader 2025 AIDS targets.”
Methodology: HIV prevention R&D investment figures are collected annually by the Resource Tracking for HIV Prevention R&D Working Group through an email survey. For the present report, the Working Group reached out from February to June 2020 to 215 funders in the public, philanthropic and commercial sectors. Two different types of resource flows were tracked: investments, defined as annual disbursements by funders; and, when available, expenditures, defined as resources directly spent on R&D activities by funding recipients. More information about the report methodology is at www.hivresourcetracking.org/about/methodology.
###
About the Resource Tracking Working Group: In its 16th annual report, the Resource Tracking for HIV Prevention Research & Development Working Group (“Working Group”) documents research and development spending for the calendar year 2020 and analyzes funding trends spanning twenty years. The Working Group is led by AVAC in partnership with the International AIDS Vaccine Initiative and UNAIDS.
JIAS Looks at the Big Picture for HIV Vaccines
As we mark World AIDS Day this week, we recommend checking out a rich and timely supplement on HIV vaccine research and development published by the Journal of the International AIDS Society. The ten-article overview provides a look at the status of research, the context surrounding it, spotlights emerging strategies, and multiple angles on why and how vaccine R&D must forge ahead.
Even with two major disappointments in clinical trials of vaccine strategies in the last two years (the Imbokodo and Uhambo trials), we at AVAC are more committed than ever to the understanding that formed the basis of AVAC’s founding 26 years ago: a durable end to the epidemic depends on the accelerated development and ethical delivery of an HIV vaccine.
Included in the wealth of material in the supplement, AVAC cofounder Bill Snow co-authored a vision for how the HIV Vaccine enterprise can and must evolve to face today’s challenges and bring tomorrow’s solutions; AVAC’s Maureen Luba co-authored with a number of our partners HIV Prevention Today: do we still need a vaccine? A community perspective, probing the role of vaccines in the prevention landscape; and AVAC’s Jessica Salzwedal co-authored with HAVEG’s Cathy Slack and other partners in our Coalition to Accelerate and Support Prevention Research (CASPR) Shifts in UNAIDS ethics guidance and implications for ethics review of preventive HIV vaccine trials, an article on evolving ethics guidance and implications for clinical trials (see additional resources below).
In addition, Cathy and collaborators further the conversation on ethics in HIV research in a post for our P-Values blog, The Ethics Review Process – A key to sound engagement.
We encourage you to explore these articles in depth. To inform your advocacy, use this supplement, resources below, and watch this space for more resources to come, because it’s never been more important to champion the need for an HIV vaccine.
Resources on the Landscape for HIV Vaccine R&D:
- Check out this infographic: The Years Ahead in Biomedical HIV Prevention Research
- Hear a podcast: Back to the beginning: AIDS and the elusive vaccine
- Read an op-ed: Finding an HIV vaccine: Five lessons from the response to COVID-19—this op-ed by Mitchell Warren and Fatima Hassan of Health Justice Initiative takes a look at what the HIV field can learn from the search for a COVID-19 vaccine
- Watch a webinar: Update on the Uhambo Trial
- Watch a webinar: Results and implications of the Imbokodo trial
Resources on Ethical Guidance in HIV Clinical Research:
- Read an overview: Ethical Guidance in Focus
- Watch a webinar: New Ethical Guidelines for HIV prevention Trials in People: What’s changed and Why Does it Matter?
In Memory of Zena Stein
Public health, human rights and HIV prevention champion, Zena Stein died this week, at the age of 99. Zena inspired and mentored nearly everyone working in public health in South Africa and in microbicide research, and has likely knowingly or not, influenced so many of us working in HIV prevention research and advocacy today.
Zena and her husband Mervyn Susser were pioneering anti-apartheid activists, epidemiologists and public health practitioners. They ended up in exile from apartheid and eventually landed at Columbia University where they both had long, distinguished careers. Amongst other things, Zena helped “launch” the field of HIV prevention options focused on women, including the female condom and microbicide research. Truly a visionary, here is what she wrote 31 years ago — when microbicides were called “virucides”:
New Resources and Opportunities!
In this round-up you’ll find opportunities to register for a webinar on cure research and preview a new course on GPP. In case you missed them, scroll down for resources that cover a spectrum of issues crucial to the progress of HIV prevention today. We hope you’ll watch, read, learn and join the conversation!
Coming up
- Starting soon! Register for a free live session previewing AVAC’s newest GPP course for funders, sponsors and principal investigators at the Union World Conference on Lung Health, today, October 20, 12:00-12:45pm EDT. Don’t miss the live Q&A with the developers of The GPP Compliance Course from 12:30-12:45pm EDT!
- Wondering about funding for HIV cure research and how communities can engage on the issue? Register for the webinar, Investment and Engagement in HIV Cure Research: Looking Ahead on Wednesday October 27, 2021 at 10am EDT.

Integrated Products, Integrated Services
- A recent webinar, Can Fantasies Become Realities? The Quest for Multi-purpose Prevention Products, explored the science underway for combining HIV prevention with either contraception or with protection against other sexually transmitted infections. Hosted by the AIDS Foundation of Chicago (AFC) and AVAC.
- Another webinar, Integration of HIV and Sexual and Reproductive Health in the Era of ARV-Based Prevention: Findings from assessments in Kenya, Malawi and Zimbabwe featured new research from AVAC and partners showing promising approaches to reach adolescent girls and young women (AGYW) with comprehensive and integrated services for HIV and sexual and reproductive health (SRH).
Pushing the Frontier of R&D
- A new report, Situation Analysis Report on Domestic Resource Mobilization for Health Research and Development in Africa by WACI Health, is essential reading in the movement to increase domestic funding among African countries for health research and development. And watch a recent recorded discussion on the issue, Civil Society Round Table Discussion on Domestic Resource Mobilization for Health Research and Development, led by WACI Health and partners.
- AVAC’s Stacey Hannah and Mitchell Warren talk about the state of HIV vaccine research and its relationship with COVID-19 vaccine research in an op-ed published in Spain’s Scientific News and Information Service. Read Initiatives to accelerate the long and difficult search for an HIV vaccine for an overview of how these two major goals in public health have spurred innovation, and how this can and must continue.
- AVAC’s new fact sheet on Evolving Designs for HIV Prevention Trials outlines important trial designs used today, those under consideration for future trials, and the context informing it all. The fact sheet also spotlights a suite of trials testing next-generation trial designs for next-generation PrEP.
- The role of gender and diversity in health research and development was the centerpiece of a webinar early this month, moderated by AVACer Nandi Luthuli. Check out the recording of The Diversity and Gender Equity in Health R & D Webinar. Hosted by SAHTAC, PATH and Campaigning for Cancer.

Many Angles on the Ring
- Read The Dapivirine Vaginal Ring: Gone far and far to go to learn what’s at stake for women and the ring in Zimbabwe, the first country where the Dapivirine Vaginal Ring has reportedly been approved. The blog by journalist and advocate Anna Miti explores what must happen next in Zimbabwe and beyond to make the Ring a reality in women’s lives.
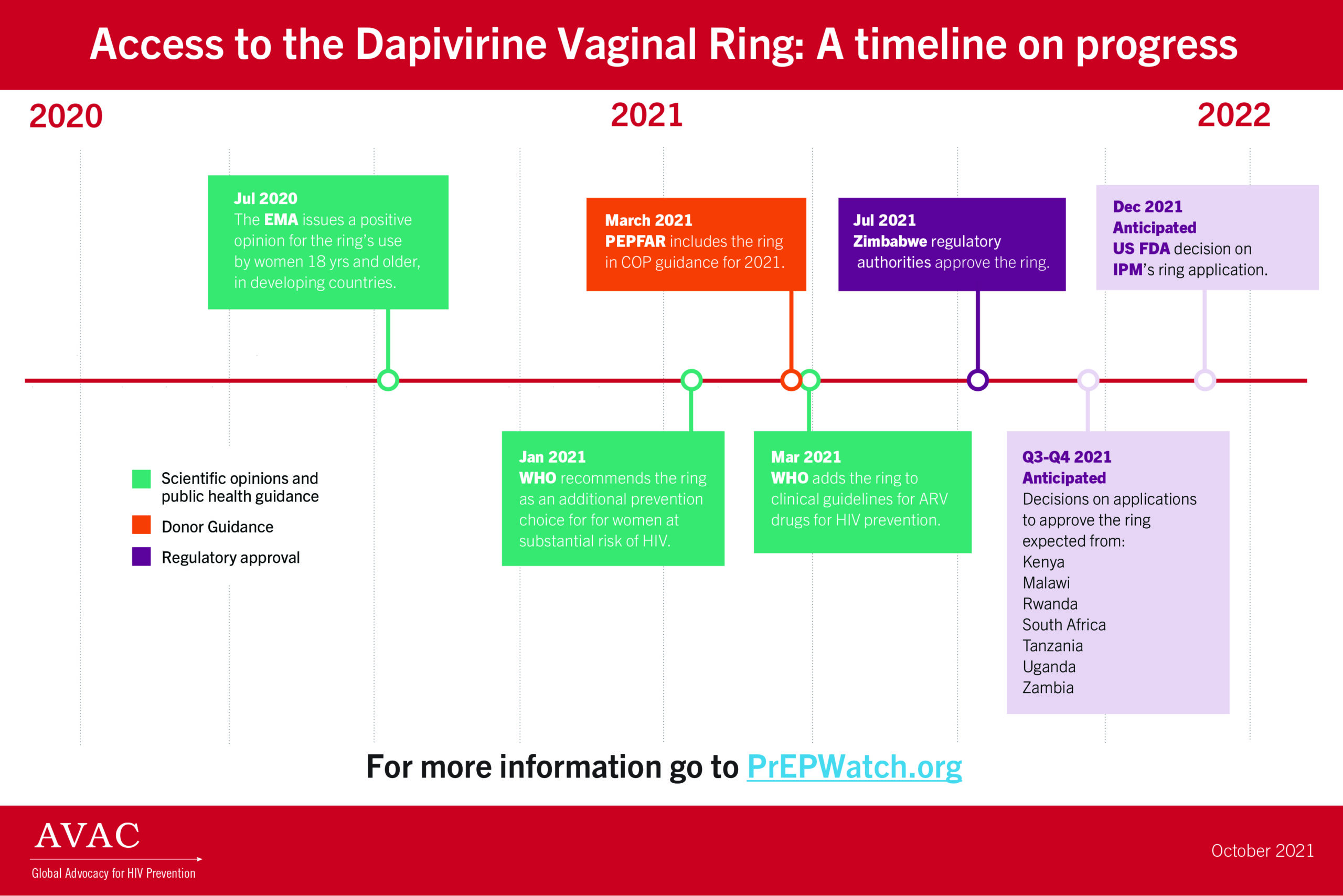
- This infographic, Access to the Dapivirine Vaginal Ring: A timeline on progress, marks key points in the journey to make the ring available.
- Learn more about the Dapivirine Vaginal Ring, the research and evidence behind it, and the advocacy needed for rollout on our dedicated ring page on PrEPwatch.org. And consider signing up to receive the PrEP Ring Quarterly Digest, a quarterly update on efforts to make the ring available, developed by AVAC and FHI 360.
Future Prospects for New Vaccines Against Sexually Transmitted Infections
This review provides an update on the need, development status, and important next steps for advancing development of vaccines against sexually transmitted infections (STIs), including herpes simplex virus (HSV), Neisseria gonorrhoeae (gonorrhea), Chlamydia trachomatis (chlamydia), and Treponema pallidum (syphilis).
HVTN 705 Vaccine Trial Ends—Join Sept 9 webinar to discuss next steps for field
Today Johnson & Johnson and partners announced that the Imbokodo study, a large-scale HIV vaccine proof-of-concept trial also known as HVTN 705/HPX2008, did not significantly reduce the overall risk of HIV acquisition among over 2,600 women in five sub-Saharan African countries. The Adenovirus26-based mosaic vaccine regimen was shown to be safe, but it did not meet pre-defined criteria for efficacy to warrant moving forward for longer follow-up. A companion study, the Phase III Mosaico trial, will continue.
Read AVAC’s full statement about the results and what needs to happen next.
Register for a global webinar, scheduled for September 9 at 10am ET to discuss this development and the implications for the HIV vaccine field.
We hope you will bring your questions and your passion. HIV continues to be a global threat. The field can and must learn from these trials and from the success of the COVID-19 vaccine enterprise, to explore new HIV vaccine strategies and bring a lasting end to the epidemic.
