AVAC has several new resources covering a gamut of cutting-edge issues for the field. An up-close look at the science covered at CROI; a handy snapshot of multipurpose technology (MPTs) moving through the research pipeline; a new infographic on “time to market” for HIV prevention products furthest along in development; and a special publication of Good Participatory Practice fitted to address COVID-19 trials. Read on for details and links for these timely resources.
Time to Market Infographic
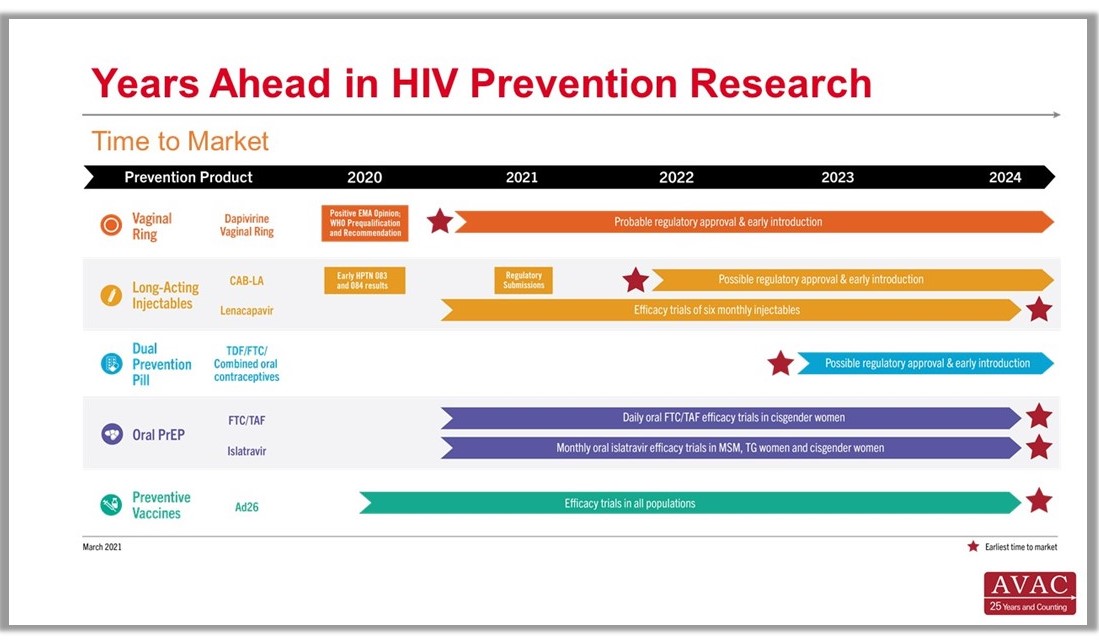
Years Ahead in HIV Prevention Research: Time to Market – The latest addition to our extensive infographic library is this new timeline showing the potential time points when the next-generation of HIV prevention options might find their way into new programs. This new graphic complements the HIV Px Research, Development and Implementation pipeline snapshot and The Years Ahead in Biomedical HIV Px Research trials timeline.
MPTs Making Headway
Advocates’ Guide to Multipurpose Prevention Technologies – Check out this guide to learn about four areas ripe for advocate involvement and get a snapshot on the status of MPT research and development, and data on investments.
Good Participatory Practice in the Age of COVID-19
Essential Principles & Practices for GPP Compliance: Engaging stakeholders in biomedical research during the era of COVID-19 – This guide to support stakeholder engagement in COVID-19 research is built from the Good Participatory Practice Guidelines for Biomedical HIV Prevention Trials (GPP). This new document responds to needs expressed by both researchers and advocates as COVID-19 research progresses with unprecedented speed and urgency. To mark the launch of this new document, AVAC hosted a webinar earlier today, which included diverse perspectives on the importance of GPP within COVID-research and beyond. Watch the recording here.
CROI in Focus
The Personal is Planetary: CROI and COVID one year on – This blog by AVAC’s Emily Bass gives context and perspective on the science and advocacy that defined the Conference on Retroviruses and Opportunistic Infections in 2021. From a call for vaccine equity to a deep dive into the findings on cabotegravir as long-acting injectable PrEP, read Bass’s blog for a picture on where the science and advocacy is moving.
We are also happy to report that, in response to community requests, CROI organizers have agreed to make all recorded content from the meeting available on April 15—five months earlier than initially planned. And, if you missed it, check out the recordings from the Daily Research Updates for advocates on AVAC’s special CROI page.
