A look at the number of participants in vaccines trials in 2019 according to trial phase.
Vaccines Trial Participation in 2019
HVAD 2019: Vaccine science needs your support!
[UPDATE: Slides and recordings from both webinars are now available. Links are provided below.]
HIV Vaccine Awareness Day (HVAD) 2019, on May 18, comes with promising headlines about advances in potential vaccines for HIV and other diseases that imperil public health. But, 2019 has also seen outbreaks of a highly infectious, vaccine-preventable disease, the result of misinformation and fear being spread by anti-vaccine campaigners.
This HVAD, AVAC has an updated toolkit of resources for translating HIV vaccine research with a renewed sense of urgency, and two dedicated hashtags to rally the call on social media: #HIVvaccineAware and #HVAD2019. We hope you’ll join the conversation — with the updated HVAD 2019 toolkit and our upcoming webinars (below and online)!
Explore all of our updated HVAD resources:
- HIV Vaccines—Key Messages lays out advocacy priorities and important updates from the field for the year ahead.
- HIV Vaccines, An Introductory Factsheet provides basic information on concepts and trials in vaccine research.
- Vaccines 101 slide deck reviews basic concepts and the status of research and development.
- HIV Vaccine R&D in 2019 slide deck provides this year’s updates on the state of the science and clinical trials.
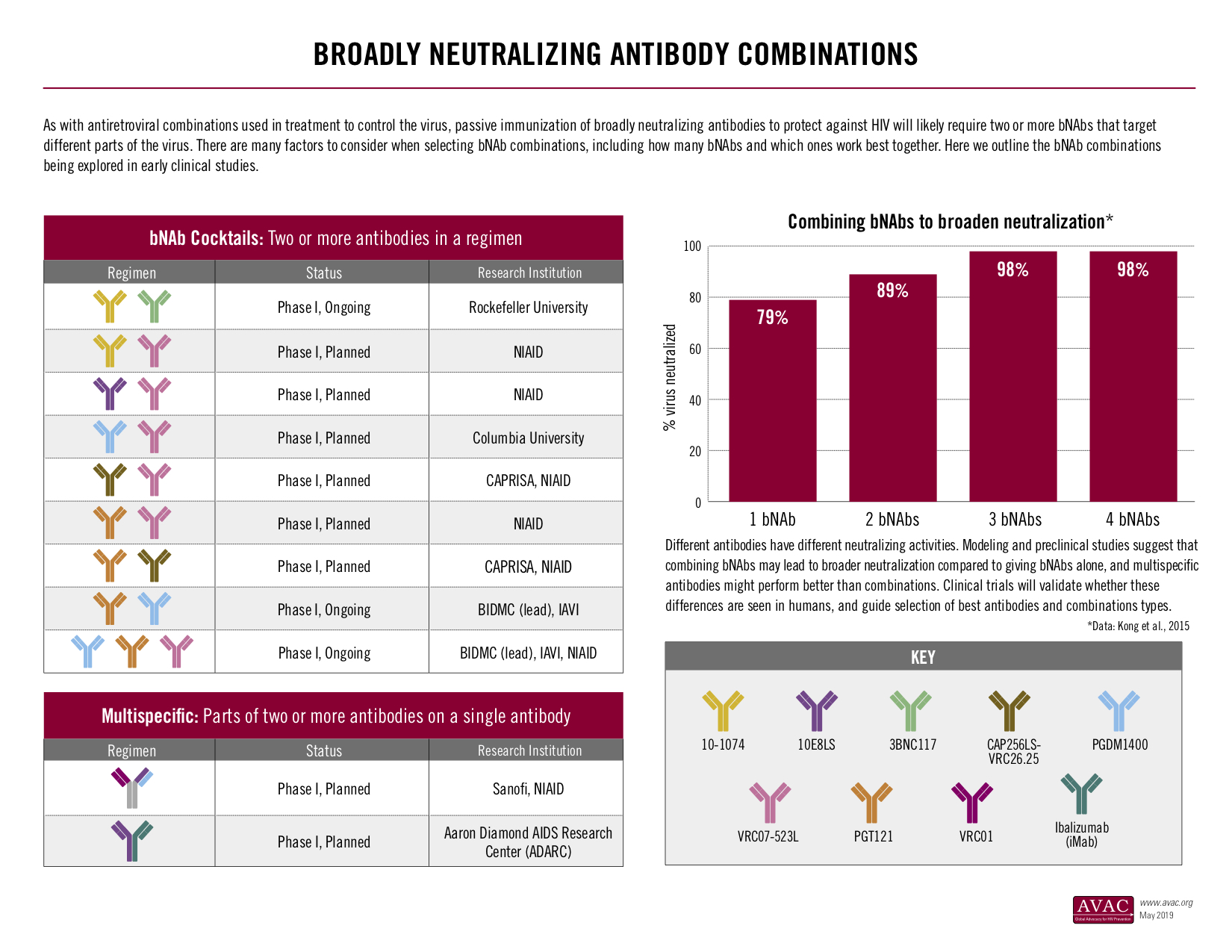
- A suite of infographics, including updates on the Vaccine Efficacy Trials Pipeline; HIV-Specific Neutralizing Antibodies: Targets and research status; HIV Vaccine Trial Participation in 2019 and others that provide a visual overview of the HIV vaccine research landscape.
One of AVAC’s HVAD Toolkit infographics showing bNAb combination research.
We also hope you will join two upcoming webinars:
- On May 16, Mary Marovich, the Director of the Vaccine Research Program at the Division of AIDS at the National Institute of Allergy and Infectious Diseases, and long-time HIV vaccine research advocate and community leader, Mark Hubbard, discussed the current landscape of vaccine research. Recording: YouTube / Audio / Introductory Slides / Mary Marovich’s Slides
- On Thursday May 23, 9am ET, Heidi Larson, Director of the Vaccine Confidence Project will discuss the implications of vaccine hesitancy and other issues along with Laura Lopez Gonzalez, deputy editor of South Africa’s Bhekisisa Health Journalism Centre.Recording: YouTube / Audio / Slides
Resources like these are essential for public understanding and support for vaccine research. Vaccines are not simple products. They require sustained investment to develop, they can be challenging to manufacture, and just as challenging to explain to potential users.
This HVAD, join us in thanking the ongoing dedication and ingenuity – of scientists, trial participants and community advocates – to find a vaccine against HIV, and let us renew our commitment to advance public understanding and support for vaccine research, development and delivery.
Two Themes and Two Webinars for this Year’s HVAD
[UPDATE: Slides and recordings from both webinars are now available at www.avac.org/hvad.]
The field is on the brink of yet another HIV Vaccine Awareness Day – next Saturday, May 18. To mark the day, we hope you will join us for webinars on May 16 and on May 23 to explore the two themes at the top of our minds here at AVAC this HVAD.
First, of course, we’re excited by the momentum and promise in HIV vaccine research. Three HIV vaccine efficacy trial programs are now underway, including, for the first time in our field, a potential path to licensure of a vaccine.
On the other hand, we’re alarmed and disheartened by a global rise in what is sometimes called vaccine hesitancy, marked by measles outbreaks and a comeback of a disease almost eradicated through a vaccine.
On Thursday, May 16, 9am ET, Mary Marovich, the Director of the Vaccine Research Program at the Division of AIDS at the National Institute of Allergy and Infectious Diseases, and long-time HIV vaccine research advocate and community leader, Mark Hubbard, will provide their perspectives on the current vaccine landscape, the advocacy priorities and what should be on all of our minds as this exciting science progresses. Register here.
On Thursday, May 23, 9am ET, Heidi Larson, the Director of The Vaccine Confidence Project at the London School of Tropical Medicine and Hygiene will discuss vaccine hesitancy and its implications across global health. We’ll also be joined by colleagues at Bhekisisa, the health journalism center of the Mail & Guardian newspaper in South Africa, who will share perspectives on broader vaccine issues, especially as they play out in the media and affect the AIDS response. Register here.
Finally, watch this space! In the coming days, AVAC will provide you with our annual HVAD Toolkit of up-to-date materials and infographics to help translate HIV vaccine research in 2019 – and prepare for the future. If you need any of these ahead of time, please reach out!
Research Literacy on a Plate: In-person HIV vaccines update for Zambian advocates
Daisy is Communications Advisor, AVAC, and based in Kenya. Chilufya is a passionate advocate for biomedical HIV prevention and research and alumnus of the AVAC Fellows program.
What does the “HVTN” in HVTN 702 stand for? And why do some trials, such as the mosaic vaccine trial HVTN705/HPX2008, carry two interchangeable tags? Also, what’s a mosaic vaccine, and why was it developed? Where are the studies happening, and when can we expect results?
Research literacy for HIV prevention advocates involves clarifying these types of questions. Current and accurate knowledge about clinical trials allows an advocate to engage confidently in the clinical trial ecosystem, which includes researchers, communities, ethics boards, and even funders. A recent gathering was a reminder of how effective it can be to conduct these sessions in an informal, low-tech setting, to invest in their knowledge of HIV prevention science.
At an early evening gathering in February, AVAC staffers joined a group of 20 advocates in Lusaka, Zambia for a research literacy session on HIV vaccines, in particular the three HIV vaccine efficacy trials planned or underway in Africa: HVTN 702, HVTN 705/HPX 2008, and the PrEPVacc trial.
Gathered together around a table in the relaxed restaurant environment of a Lusaka hotel, with snacks in hand, attendees turned to paper handouts (no tech) and gave their attention to the two-hour training. The session covered a basic introduction to vaccine science and the research process, the naming of the clinical trials, trial populations and study timelines. Attendees delved into the composition of HIV vaccines, posed questions, and learned things like how various vectors deliver immunogens, often with adjuvants added to improve the body’s immune response to a candidate vaccine.
The group discussed why it’s been so challenging to develop a highly efficacious HIV vaccine, and the exciting work in the field of broadly neutralizing antibodies, or bNAbs for short. Interaction, questions, answers, and some digressions—the group detoured into a conversation about why young women in East and Southern Africa are especially vulnerable to HIV—all of which encouraged vibrant engagement with the material.
In addition to the science, discussions included a look at other issues relevant to clinical trials for HIV prevention. Advocates in Africa play a critical role in two key areas: pushing their governments to allocate more resources for research, and helping community stakeholders understand increasingly complex trial designs.
Participants declared the after-hours session a hit. They found the discussion on vaccine research “very informative” and “beneficial and interactive.”
Clever Chilende of the Treatment Advocacy and Literacy Campaign (TALC) said, “The environment was very relaxing and enabled maximum participation!”
Chilende is also a Community Advisory Board member for several HIV prevention clinical trials in Zambia. He said he would use the information gained from the training “to create awareness among our members in the community”, and called for further sessions to “look at how the research sites are engaging with various stakeholders in order to have their buy-in and improve community participation in the process.”
Other topics attendees proposed were: Ethics in research; clinical trials involving adolescents; issues around vaccine-induced seropositivity; research on hormonal contraception and HIV risk; and community preparations for vaccine trials. All participants said “yes!” to attending a similar training in the future, and most of them said community engagement and partnership were cardinal to fostering support for research.
The same week in Lusaka, AVAC staff joined a meeting of Counselor Supervisor Officers (CSOs) working at IAVI-affiliated clinical research centers. This meeting provided an opportunity for a more formal research literacy talk covering HIV prevention trials broadly: vaccines, ARV-based prevention, antibody-mediated prevention, as well as the recently completed Evidence for Contraceptive Options and HIV Outcomes (ECHO) study. The upcoming open-label trials that will assess the use of oral PrEP and dapivirine ring in pregnant women (MTN-042 DELIVER, and MTN-043 B-PROTECTED), as well as the efficacy trials pipeline were discussed.
Dr. William Kilembe, Project Director of Zambia-Emory Research Initiative in Tuberculosis and TB/HIV (ZEHRP), joined both the advocates’ and CSO sessions. ZEHRP is one of the HVTN 705/HPX2008 vaccine trial sites in Zambia, and Dr Kilembe’s contribution grounded the discussion in details about how the study is being conducted, achievements, challenges, and community perceptions.
In case you’re still puzzling over the questions at the top:
- “HVTN” stands for the HIV Vaccine Trial Network, the world’s largest publicly funded multi-disciplinary international collaboration facilitating the development of vaccines to prevent HIV/AIDS. The network collaborates with research institutions in over 30 cities on five continents, and is currently conducting the HIV vaccine efficacy trials HVTN 702 and HVTN 705/HPX2008.
- HVTN 705/HPX2008, a clinical trial also known as Imbokodo, is underway in Malawi, Mozambique South Africa, Zambia and Zimbabwe, testing a mosaic HIV vaccine candidate designed by the pharmaceuticals company Janssen to protect against the most common variants of HIV. Janssen is also sponsoring the trial, whose double tag simply represents the HIV Vaccine Trials Network’s (HVTN 705) and Janssen’s (HPX2008) naming systems.
- The AVAC infographic: The Years Ahead in Biomedical HIV Prevention, gives a snapshot of HIV prevention efficacy trials, their products, locations, and timelines.
In-person research literacy trainings will continue to be part of AVAC’s regular research translation and advocacy offerings, which include publications, the Px Wire newsletter, primers and fact sheets, infographics, Advocates’ Network updates, webinar series, P-Values blog, Px Pulse podcast, media cafés for science journalists, and a new online learning and networking platform known as Engage!
For opportunities to participate in short trainings in your area, or for assistance in organizing one, contact us at [email protected].
Community Engagement and HIV Prevention Research
This December, Px Pulse features a gripping, tripartite conversation between activists Morenike Giwa-Onaiwu, Stacey Hannah and Jeremiah Johnson about what their long histories fighting for community engagement in HIV prevention research have taught them, and how these lessons can be applied today, and in the future. Tune in to hear three fierce voices with fresh perspectives on how to continue designing trials, and engaging communities, in today’s landscape of expanded, but inadequate, prevention choices.
With daily oral PrEP, VMMC, partner testing and treatment that leads to virologic suppression available as potent biomedical tools, along with condoms and a range of other structural interventions, clinical trials of biomedical HIV prevention strategies to block sexual transmission are more complex, in terms of design and conduct, than ever before.
Listen to this episode of Px Pulse on iTunes or at www.avac.org/px-pulse to learn why this conversation is at a critical moment and how to manage the opportunities for an innovative and collaborative effort with the research community.
Also, in case you missed it, check out the latest issue of Px Wire, which is hot off the presses. Our year-end edition offers 10 questions for activists to galvanize their work in the year ahead. We consider the future of NIH funding for HIV prevention and its research priorities, anticipated results from the ECHO trial, what’s next for the dapivirine ring and much more! The centerspread visualizes a single time frame for trial results and critical targets for incidence reduction and scale up of primary prevention-an essential perspective for the work ahead.
Finally, please don’t forget, your support makes our work possible. Help us continue with a year-end donation at www.avac.org/donate. You can also use smile.amazon.com for your online shopping and select AVAC as your charity of choice. A portion of your purchase price is donated to AVAC—at no additional cost to you!
Happy listening and reading, and happiest of holidays!
One Timeline, Two Stories, One Message: Putting trials and targets together
One problem with HIV prevention agendas is that they either live in an eternal present or in a far-off future. It’s “work with what we’ve got, which is condoms and VMMC and a little bit of PrEP”, or it’s “nothing can change without an AIDS vaccine”. The future depends on using what’s available, better and more widely, without ever losing sight of what’s in the pipeline.
As the figures below show, in the very same timeframe that the world will miss its critical target for incidence reduction and scale-up of primary prevention, several trials will release results that could change the future. 2020 will be a time of hope and reckoning. But only if the two stories start to be told as one.
The Latest Issue of Px Wire! What to Watch in 2019
As 2018 winds down, we’re struck by the many moments, and movements, in the past year that have depended on listening, without bias and also without loss of conviction. From a bold activist challenge in an elevator, to an array of young women speaking their truths about HIV prevention—the future has hinged on being willing to listen, and on demanding to be heard.
In that spirit, our year-end edition of Px Wire offers 10 questions for activists to pose, with curiosity and conviction, in 2019. What answers do you want, what do you hear, what needs to happen next? We’ll be listening!
Our questions take on the upcoming announcement of how future NIH funding of HIV research will shape biomedical prevention, the anticipated results of the ECHO trial looking at how different contraceptive options impact women’s risk of HIV, the future of the dapivirine vaginal ring and much more.
In our centerspread, we provide a visual for uniting biomedical prevention research and implementation—a necessary fusion for our work in the coming year, and beyond.
Also necessary: your continued support. AVAC depends on your contributions of work, ideas and, yes, funds for our work! We appreciate your support in one or more of the following ways:
- Donate: Visit www.avac.org/donate.
- Amazon Smile: Shop at Amazon.com? Visit smile.amazon.com and select AVAC as your charity of choice and a portion of your purchase price is donated to AVAC—at no additional cost to you!
- US Combined Federal Campaign: If you are a US government employee, support our mission through the Combined Federal Campaign, CFC #12308.
Many thanks for your continued support, partnership and inspiration.
HIV Prevention Research and Demonstration Sites in South Africa
This map demonstrates the breadth of HIV prevention research and demonstration projects in South Africa by site and type (e.g. daily oral PrEP demo projects, ARV-based rings, long-acting injectable PrEP, preventative Vaccines, antibodies, hormonal contraceptives). This map was developed by Wits RHI with support from AVAC as part of the Coalition to Accelerate and Support Prevention Research. This graphic first appeared in AVAC Report 2017: Mixed messages and how to untangle them.
Global HIV Prevention R&D Investment by Technology Category, 2000-2017
In 2017, reported funding for HIV prevention R&D decreased by 3.5 percent (US$40 million) from the previous year, falling to US$1.13 billion. The full report, HIV Prevention Research & Development Investments 2017: Investing to end the epidemic, is available for download. And all the graphics are available as well.
HIV Prevention R&D Trial Participants by Region in 2017
Participation of volunteers and the engagement of communities in which trials take place is essential to conducting HIV prevention research. In 2017, there were nearly 600,000 participants in HIV prevention research trials globally, mostly originating from sub-Saharan Africa, Europe, North America and Asia. A majority of participants were enrolled in research investigating TasP and PrEP, and while there are trials aimed specifically at men who have sex with men (MSM), transgender individuals and people who inject drugs, most of the studies do not specify the need to include members of key populations.
The full report, HIV Prevention Research & Development Investments 2017: Investing to end the epidemic, is available for download.