On this World AIDS Vaccine Day, the field of global health faces a much-changed world. The extraordinary successes in COVID-19 vaccine development, which stemmed from the scientific knowledge and networks created by the HIV response, have advanced vaccine development by leaps, with innovation, commitment and coordination that accelerated the research and development process at unimaginable speed. The pandemic also exposed the entrenched barriers to vaccine access at a scale never seen before. Misinformation, stigma, greed, and the humbler problems of coordination and planning have hampered delivery of COVID vaccines, just as they have HIV prevention.
The response to COVID-19 makes clear, the world must do better. Scientific advances and equity must go further to deliver HIV prevention and a future HIV vaccine. However, in the past two years, results from major HIV vaccine trials have both upended what we know and reframed the questions we must ask. We need to act with urgency to develop new and faster models for advancing HIV vaccine science that can adapt quickly to what is learned. And we need to continue to push new models for equitably delivering the fruits of that science.
This HVAD, AVAC is kicking off a series of conversations to reframe and reenergize the search for an HIV vaccine.
 In 2020, AVAC saw the connections between COVID-19 and HIV, and outlined their implications in Five “P”s to Watch.
In 2020, AVAC saw the connections between COVID-19 and HIV, and outlined their implications in Five “P”s to Watch.
In 2022, those insights remain central to what lay ahead, and we’ve built on them as we consider the state of the HIV vaccine field today. We’re bringing together some of the most creative minds in the field—advocates, researchers, policy makers and vaccine funders – to explore four angles we’re watching as we make progress towards an HIV vaccine.
Webinars and New Resources
Platforms & pipelines for developing new approaches to HIV vaccine research.
 COVID-19 ushered in a new “Golden Age” in research on vaccines using a previously unproven delivery platform – messenger RNA (mRNA). mRNA vaccines hit the target in COVID, but will they work in HIV? What antigen or combination of antigens should it deliver to be effective? Join this webinar or use this fact sheet to learn more about what researchers have learned, what remains to be discovered about mRNA and HIV vaccines, and about the HIV mRNA HIV vaccine studies now underway.
COVID-19 ushered in a new “Golden Age” in research on vaccines using a previously unproven delivery platform – messenger RNA (mRNA). mRNA vaccines hit the target in COVID, but will they work in HIV? What antigen or combination of antigens should it deliver to be effective? Join this webinar or use this fact sheet to learn more about what researchers have learned, what remains to be discovered about mRNA and HIV vaccines, and about the HIV mRNA HIV vaccine studies now underway.
Webinar
Wednesday, May 18 with Bart Haynes (Duke), Nina Russel (Bill & Melinda Gates Foundation) and Ntando Yola (DTHF).
Recording and Slides: YouTube / Nina Russell’s Slides / Bart Haynes Slides
Processes that offer innovation on the traditional phase I/II/III approach to research.
 Biomedical research has evolved more rapidly in recent years than in any time in human history. New bioengineered platforms and products are changing the ways diseases are treated and prevented. And new global commitments to sharing information and data are finally moving the needle toward making research a truly global enterprise. In many ways, though, HIV vaccine trial design remains stuck in the 20th century.
Biomedical research has evolved more rapidly in recent years than in any time in human history. New bioengineered platforms and products are changing the ways diseases are treated and prevented. And new global commitments to sharing information and data are finally moving the needle toward making research a truly global enterprise. In many ways, though, HIV vaccine trial design remains stuck in the 20th century.
New approaches to research such as experimental medicine vaccine trials (EMVTs) offer the prospect of answering crucial questions safely and quickly. But the commercial, legal and regulatory frameworks are not designed to move HIV vaccine research through the pipeline with greater certainty, ease and speed. And community engagement models for these next-gen research approaches are still in development. Join us to discuss the opportunities and challenges of new approaches to vaccine research, and how advocates can help maximize the potential of a 21st century HIV vaccine research agenda.
Webinar
Tuesday May 24, 2022 with Gail Broder (HVTN), Pontiano Kaleebu (MRC/UVRI & LSHTM Uganda Research Unit) and Robin Shattock (Imperial College).
Recording and Slides: YouTube / Robin Shattock’s Slides / Gail Broder’s Slides / Pontiano Kaleebu’s Slides
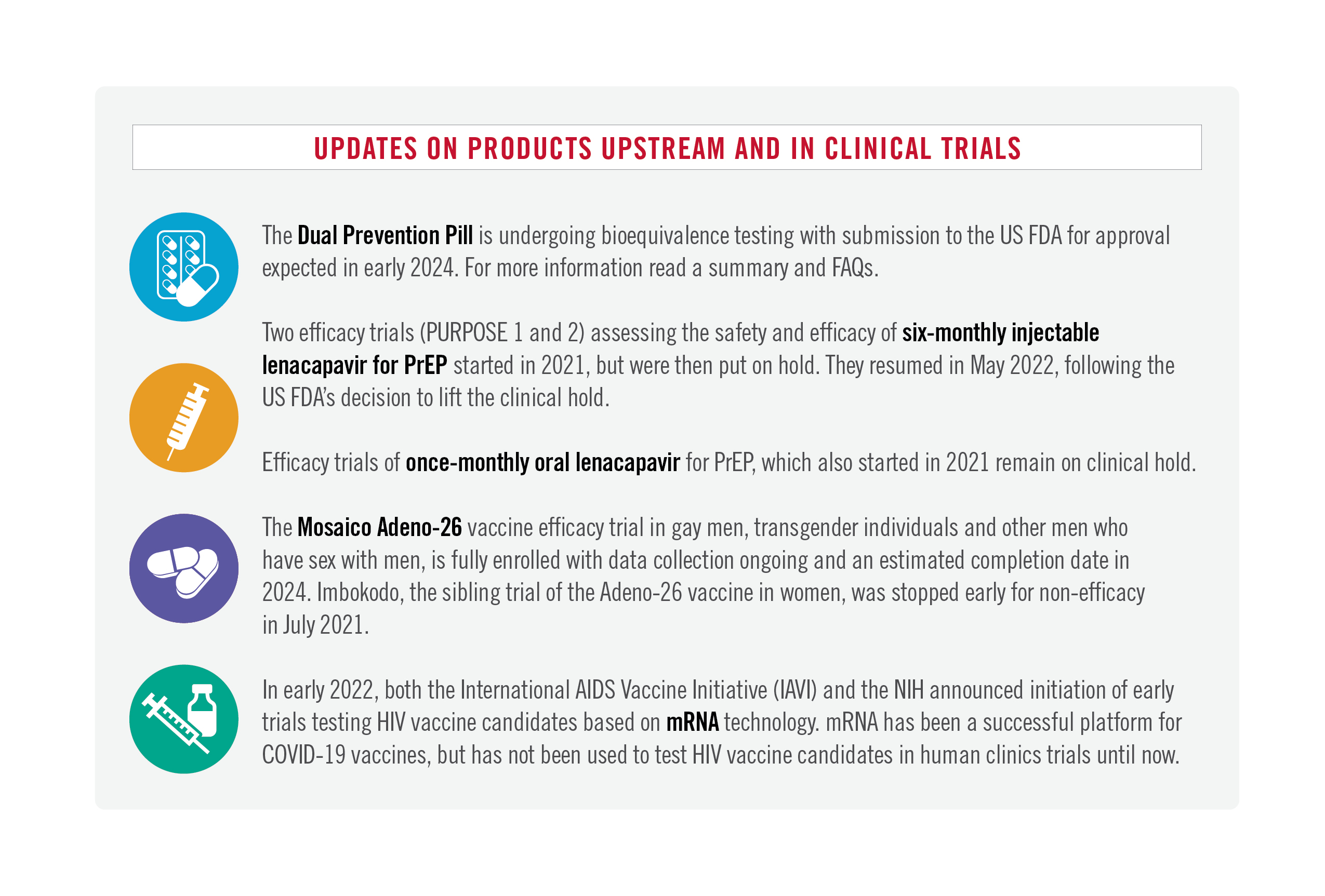
Prospects for HIV vaccine products in development, and for new approaches that may need more support.
Thanks to the efforts to tens of thousands of volunteers, researchers and advocates, the world has learned infinitely more about the human immune system, vaccine science and HIV than was known when HIV Vaccine Awareness Day was first commemorated twenty-five years ago, in 1997. Given the current state of HIV vaccine science, the broader HIV prevention landscape, and what’s been learned through COVID, how should HIV vaccine research move into the future? How can we best use that hard-earned knowledge to make choices about HIV vaccines in development now, and chart a course for which products on the horizon have the best chances of achieving their ultimate goal?
Webinar
Tuesday May 31, 2022 with Galit Alter (Harvard), William Kilembe (ZEHRP), Ethel Makila (IAVI) and Dale Hu (NIH).
Recording: YouTube