October 22, 2025
This issue provides a range of maps to help orient the field on critical HIV prevention activities: the status of delivering injectable cabotegravir (CAB for PrEP); funders and countries on track for early introduction of injectable lenacapavir (LEN for PrEP); and where new Phase 3 efficacy trials testing MK-8527 as a monthly pill for PrEP are taking place.
Key Takeaways
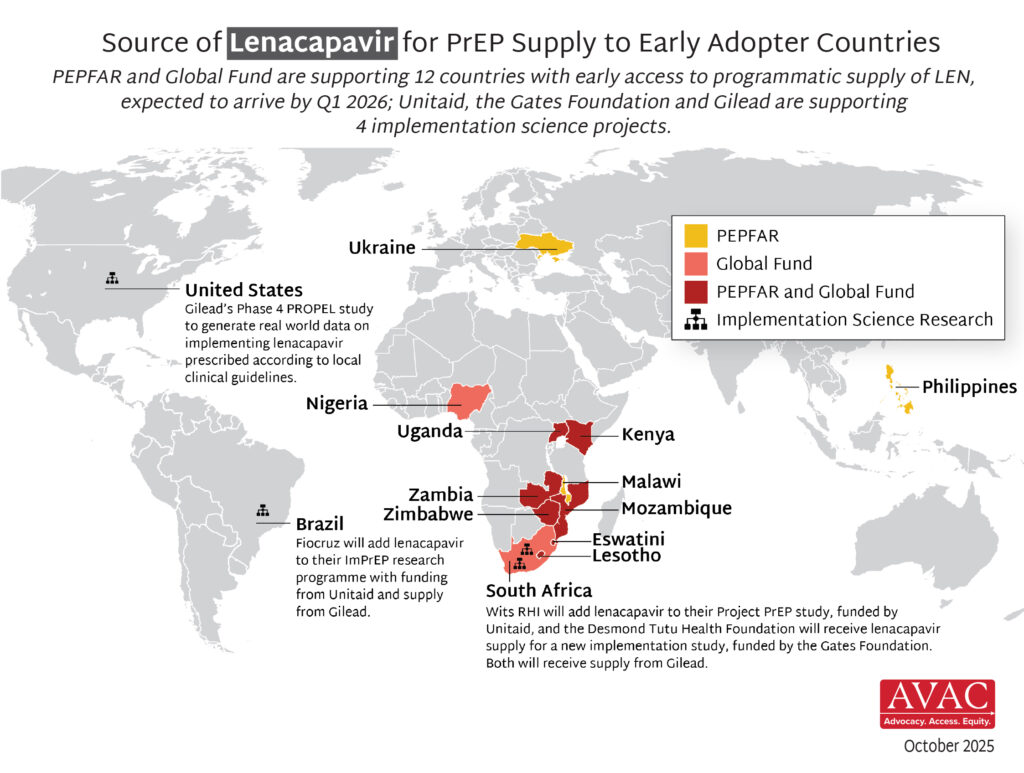
- Supply of LEN is due to begin arriving in countries in late 2025 with service delivery planned to start in early 2026.
- The Global Fund, with support from CIFF, and PEPFAR have jointly committed to reaching up to two million people with LEN for PrEP over three years.
- Seventeen countries are hosting sites for the Phase 3 efficacy trials of a monthly PrEP pill, MK-8527, being developed by Merck. Merck announced the launch of the Phase 3 trials at IAS 2025 in Kigali. MK-8527 was found to be safe and well-tolerated in Phase 2 clinical trials.
- Merck’s commitment to stakeholder engagement to date contributes an important model of Good Participatory Practice (GPP) to the field, by putting global advocates at the forefront of planning for the program and trial design. Merck has expressed a commitment to sustain this vital engagement throughout the program and next steps.