This resource package directs prospective applicants of the Fellows Program to online resources on HIV prevention research and general information on HIV and AIDS. For more information on the Fellows program, visit www.avac.org/fellows.
Resources & Recommended Reading for Prospective 2022 Applicants for the Fellows Program
Frequently Asked Questions for Host Organizations
This resource is a FAQ document for prospective host organizations of the Fellows Program. For more information visit www.avac.org/fellows.
AVAC Advocacy Fellows Quick Guide for Applicants
Brief two-pager outlining the AVAC Advocacy Fellows program. More information available at www.avac.org/fellows.
Including Pregnant and Lactating Populations in HIV Prevention Research
Pregnancy and the post-partum period after giving birth are times of heightened HIV risk. Cisgender women are two to three times more likely to acquire HIV during pregnancy and four times more likely post-partum than otherwise. Women who acquire HIV during pregnancy have an 18 percent chance of transmitting HIV to their newborn, which goes up to a 27 percent chance if they acquire HIV while breastfeeding.
Yet, HIV prevention options for pregnant and lactating populations (PLP) are limited, and their inclusion in research inadequate and hence evidence gaps can be seen across the research landscape for new prevention products. Most biomedical HIV prevention research excludes PLP, and those who become pregnant during a trial are typically stopped from further use of the study drug. PLP include cisgender women, transgender men and those who identify as gender non-binary who are able to get pregnant. For transgender and gender-diverse pregnant people, evidence gaps are even further magnified.
The exclusion of Pregnant and Lactating People (PLP) from research results in:
- A lack of data on dosing and maternal and fetal safety
- Limitations around prescribing potentially beneficial interventions
- Exclusion from potential direct benefits of research participation
- Delays and discrepancies in health policies and programs
There is growing consensus for the greater inclusion of PLP in HIV prevention research as a public health ethical imperative, but action and a paradigm shift is needed from a variety of stakeholders to promote ethical inclusion rather than presumptive exclusion of pregnant women from clinical drug trials.
“Excluding pregnant women from clinical trials doesn’t eliminate risk, it simply shifts the risk from research studies to the doctor’s office, where pregnant women receive treatments rarely supported by robust data about how they will respond and whether the drugs are effective in pregnancy.” – Dr Anne Lyerly, PHASES, Source: Undark 2020
The Pregnancy and HIV/AIDS: Seeking Equitable Study (PHASES) Project guidance identifies conceptual shifts for the ethical framing of research as shown here.
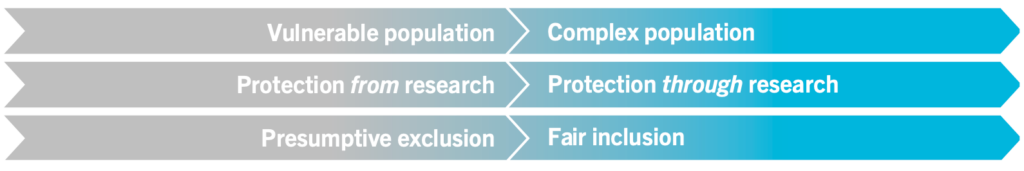
Ethical Shifts in the Framing of Research in PLP (PHASES, 2020)
The resources below provide background on the issue, updates on progress toward the inclusion of PLP in HIV prevention research and explore advocacy to accelerate this progress.
- Advancing HIV Prevention Research in Pregnant and Lactating People (PLP): Think Tank Report & Action Plan — The work of the AVAC/PHASES think tank to advance HIV prevention research with pregnant and lactating people was importantly grounded in a trio of conceptual frameworks.
- Pregnancy and Neonatal Safety Outcomes of Timing of Initiation of Daily Oral Tenofovir Disoproxil Fumarate and Emtricitabine Pre-exposure prophylaxis for HIV Prevention (CAP016): An open-label, randomised, non-inferiority trial — Appearing in the Lancet HIV, this research provides much-needed safety data to allow for a more informed choice during pregnancy to protect mother and baby from the long-term effects of HIV.
- Advancing HIV Prevention Research in Pregnant and Lactating Populations (PLP): Priority Advocacy Objectives and Next Steps — A four point action plan to advance the inclusion of PLP in clinical research for HIV prevention.
- An Advocate’s Guide to Research in Pregnant and Lactating Populations — A resource that provides background on the need for research in PLP and how advocates can advance inclusion
- Special JIAS Issue — Approaches to enhance and accelerate investigation of new HIV drugs in pregnancy. Guest Editors: Elaine J. Abrams, Martina Penazzato. Published in 2022.
- The History of Reproductive Justice — A video created by In Our Own Voice: National Black Women’s Reproductive Justice Agenda
- Ending the Evidence Gap for Pregnant Women Around HIV/co-infections: A call to action — Guidance for advancing HIV/co-infection research with pregnant women, issued by the PHASES project in 2020
- Research for Informed Choices: Accelerating the study of new drugs for HIV in pregnant and breastfeeding women: A call to action — A multi-stakeholder action plan for promoting the inclusion of pregnant women in HIV, issued by WHO, IMPAACT and IAS in 2021
- State of the HIV Prevention Evidence Base — Data on HIV prevention options for PLP and upcoming research presented by Dr. Lisa Noguchi at the AVAC/PHASES Think Tank in 2022
- Approaches to Enhance and Accelerate Investigation of new HIV Drugs in Pregnancy — A JIAS supplement published in 2022
- Where are the pregnant and breastfeeding women in new pre-exposure prophylaxis trials? The imperative to overcome the evidence gap — An article that outlines PLP involvement in recent and ongoing HIV PrEP trials and advocacy for further inclusion, written by Dr. Dvora Joseph Davey et. al and published in 2022
- Why Are So Few Drugs Tested for Safety in Pregnancy? — An article from the Undark independent magazine that provides a summary of the history and regulatory changes with regards to why so few drugs are tested for safety in pregnancy. Published in 2020
HIV Vaccine Awareness Day (HVAD) 2023
In 2023, AVAC and CASPR partners cast a spotlight on many issues and opportunities for HIV vaccine science. This one-pager highlights key conversations.
Language for HIV Cure
This document shares up-to-date, community-preferred terminology within cure research.
Experimental Medicine Vaccine Trials (EMVTs): Opportunities and Challenges
In 2023, AVAC and CASPR partners cast a spotlight on many issues and opportunities for HIV vaccine science. This one-pager highlights key conversations.
COVID-19 Resources for Advocates
This document is to help advocates with links to the latest information about COVID-19, including information on its prevention, transmission, relationship to HIV and research that’s underway. Many of the links below are constantly updated so please click through for the latest.
HIV Vaccines: An introductory factsheet
This is an updated introductory two-page document that describes HIV vaccines research and reviews key developments in the field.
Participant Application Form
Download the application here or click the link to the right of this page. Please note, you will need to download and fill the application on a laptop or desktop computer. It is not possible to use a mobile phone to fill out the application.
The Advocacy Navigator is an AVAC initiative combining training and mentorship to young and emerging advocates in the field of HIV prevention advocacy. The program involves three months of coursework and project development, followed by three months of “implementation” whereby Navigators implement all or a portion of their Community Advocacy Project plan with the support of their mentor. More information at www.avac.org/navigator.