AVAC joined more than 100 organizations to endorse the 2023 Blueprint for Sexual and Reproductive Health, Rights, and Justice Policy Agenda. Check it out to see recommendations to the US Executive Branch that would protect and expand access to sexual and reproductive health, rights and justice around the world. This Blueprint comes as several major threats to sexual health, rights and justice are gaining ground: the loss of abortion access and gender-affirming care in the US, and criminalization of LGBTQ people around the world.
Blueprint for Sexual & Reproductive Health, Rights & Justice
CASPR Results Bulletin
The Results Bulletin is a Monitoring, Evaluation, Results and Learning (MERL) newsletter providing updates, news and insights on results from CASPR activities, practices, trends and tools on a quarterly basis. The purpose of this newsletter is to inform and engage CASPR partners and other stakeholders about the progress, achievements, challenges and lessons learned from CASPR initiatives.
A Legacy of Impact: The power and reach of AVAC’s Advocacy Fellows
For more than a decade, alumni from AVAC’s Advocacy Fellows program have been expanding the reach of HIV prevention and advancing global health equity around the world. They are influencing research and strengthening healthcare to become more effective and equitable across the continent of Africa and beyond. A review of their career achievements shows how this unique program is contributing to the strength of a generation of leaders, and how their cumulative efforts are shaping the HIV response for the better.
Founded in 2009, the Advocacy Fellows program offers technical and financial support to individuals living in areas where HIV incidence is high, with a particular focus on African countries. The program invests in a select group of advocates who have an interest in improving the HIV response in their communities. The program pairs individuals with a community-based host organization where they pursue an 18-month advocacy project. Both the individual fellow and the host organization develop expertise and advocacy skills, gain access to influential stakeholders and networks, and receive intensive mentoring.
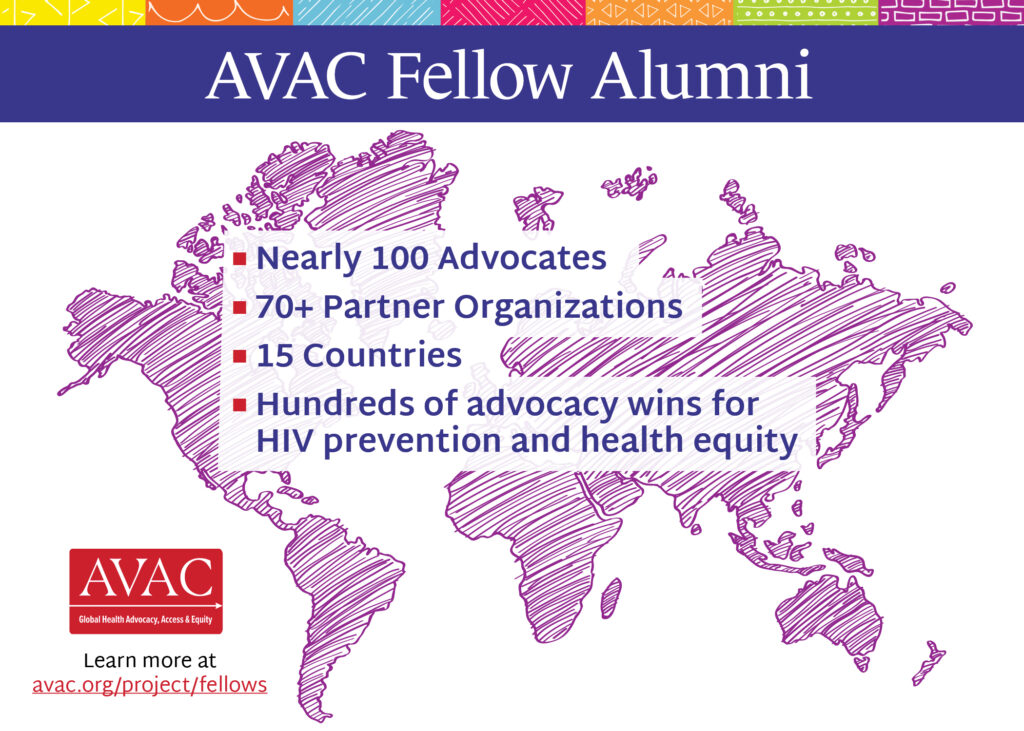
Nearly 100 advocates working with 70+ partner organizations across 15 countries have participated in the program. In that time, Fellows have:
- Founded or leads more than 85 influential organizations, coalitions and campaigns.
- Changed more than 84 national policies in support of HIV prevention or greater equity in public health.
- Won significant funding* increases for more than 83 different high-priority prevention programs and projects.
- Gained approval for over 35 innovative programs (including implementation, service delivery or community projects) and research projects.
- Led nearly 200 high-impact efforts** to create an enabling environment for HIV prevention such as rallies, media placements, and research literacy initiatives.
- Served on at least 150 high-level decision-making bodies, affecting policies, programs and funding at national and global levels.

A look at the careers of three alumni – Kenya’s Grace Kamau, Uganda’s Kenneth Mwehonge and South Africa’s Yvette Raphael – exemplifies the importance of this program in preparing future leaders, and showcases the extensive influence that alumni Fellows have on public health.
Grace Kamau joined the 2011 Fellows program with experience as a peer-to-peer educator for a sex worker advocacy organization, the Bar Hostess Empowerment and Support Programme (BHSP). She describes herself at the time as shy, uncertain of how and when to speak her mind. “I didn’t even apply for a conference scholarship because I was intimidated to travel”. As she worked on her project— raising awareness of PrEP and microbicides research among a sex worker community in Kenya, and engaging with policy makers to support the provision of PrEP to sex workers— elements of the program’s mentoring brought a seismic shift in her advocacy. “The Fellowship builds the whole person, far beyond expertise in HIV prevention, I came out a changed person.” A host of skills came together: mastering a two minute speech in case of encountering a key official in a hall, making quick follow up a standard practice, retaining command of the facts, understanding HIV science and policy trends. “Ever since I came out of the Fellows program, I have stood out. I pinpoint what needs to be said and to whom. I understand the power of my voice to speak out for sex worker rights and welfare. That confidence was built during my fellowship, and I use it to advance a movement.”
Grace’s advocacy has contributed to more than 175,000 sex workers in five countries receiving access to HIV services through the Hands Off Project, which she chairs. Her advocacy has also given voice and visibility to sex workers from 35 African countries on the need for decriminalization, access to care and other anti-stigma work in her current role as Regional Coordinator of the Africa Sex Workers Alliance (ASWA).
Kenneth Mwehonge grew up in a region of Uganda burdened with some of the highest rates of HIV found anywhere in the world. He saw one sister die, unable to get treatment and another finally gain access to life-saving drugs after living with HIV for many years. In 2014, he applied for a fellowship, work with HEPS-Uganda as his host organization. At the tine, he had only local level experience in grassroots organizing and a desire to fight for broader access to treatment. “My Fellowship with AVAC was the turning point in my career.” Kenneth said his exposure to the alumni network, introductions to decision-makers, education about HIV prevention and the research process, and AVAC’s mentoring boosted his confidence and taught him the value of mashalling evidence, working with data, and tracking progress closely. “When the fellowship began I was sometimes quite intimidated to talk to leaders, but overtime that wasn’t true anymore.” By the end of his project, Kenneth had profoundly impacted the HIV response in Uganda, designing and promoting a plan for universal viral load monitoring that was embraced by both PEPFAR and the Uganda government. His leadership and advocacy continued to grow.
Today, Kenneth is the executive director of HEPS, the organization that once hosted him as a Fellow, and it has grown into one of the most trusted civil society institutions in the country. Kenneth is also the coordinator of the Uganda Coalition for Access to Essential Medicines. He devotes significant time to mentoring emerging advocates. “It does take a lot of time, but there is so much work to be done and it is so important to see young people with fresh thinking taking the work across the finish line.” Kenneth’s leadership is also prized at the international level; he currently serves as a member of the UNAIDS Global HIV Prevention Coalition and the lead for coordinating the 2025 roadmap for prevention in Uganda and Tanzania.
“For the first time, we have options—PrEP, the ring, and cabotegravir as an injection. But advocates from my hometown, where [HIV] prevalence is 17%, don’t even know these options exist! In the years ahead I will be working to see every proven option for HIV prevention is scaled up and rolled out.”
A veteran human rights activist, Yvette Raphael joined the AVAC Advocacy Fellowship cohort of 2014, with the Centre for Communication Impact (CCI) as her host organization. As a woman living with HIV, she wanted to develop a program to mobilize young women to fight for HIV prevention. She said the fellowship taught her how to implement advocacy, create a process, and be accountable to a grant. She emerged from the program with a laser focus and has since become one of the most prominent advocates for HIV prevention on the national and global stage. To name a few of her roles: Yvette is the Executive Director of South Africa’s Advocacy for Prevention of HIV and AIDS (APHA), an organization she co-founded with two other Fellow alumni – Ntando and Brian Kanyemba. She also serves on the South Africa National AIDS Commission. She was the keynote speaker at the 2023 Conference on Retroviruses and Opportunistic Infections. In the Journal of the International AIDS Society, Yvette recently co-authored Antibodies for HIV prevention: the path forward with world renowned scientists, issuing a call to action for a research agenda focused on ensuring equitable access.
She co-founded the African Women’s Prevention Accountability Board, which engages industry, research and government on ongoing and future research and is leading efforts to advance The HIV Prevention Choice Manifesto. She has helped to shape numerous programs and campaigns aimed at reaching vulnerable populations with HIV prevention and she mentors young advocates.
“The Fellows program took a chance with an unpolished, street smart activist and turned me into a boardroom eloquent and knowledgeable advocate. It was mentorship , contacts, support from the team, and solidarity with other fellows. I look down the road and I see there’s a chance to end this epidemic, but greed and power hoarding will derail all this, without advocates who are prepared and committed. I will be among them, with young advocates I am mentoring now, and so many fellow alumni by my side.”
For a comprehensive understanding of the impact of the Fellows program in its first decade, please see the AVAC Advocacy Fellows Program Evaluation, conducted in 2020.
Significant funding*- This data comes from advocacy that won funding increases from PEPFAR, Global Fund, philanthropy, bilateral government entities, and private industry for programs with extensive reach and impact, such as PrEP for AGYW and Community-led monitoring.
High-impact efforts**- This data reflects initiatives that were covered by the media, media placements in national press, social media campaigns that went viral.
Px Wire September 2023
DPP Audience & Provider Insights for the DPP Research and Marketing Plan: Phase 1 Research Findings
This report, by M&C Saatchi World Services, AVAC, and partners, highlights learnings from Human-Centered Design Research undertaken in 2022 to understand the values and motivations of potential users and influencers of the Dual Prevention Pill (DPP) in Kenya, South Africa, and Zimbabwe.
Dual Prevention Pill: Market Preparation and Introduction Strategy
This strategy, updated in August 2023, is intended for donors, governments, implementing partners and civil society to inform priorities and planning for DPP rollout. The strategy describes activities required to build a cohesive body of evidence and recommends an approach to DPP introduction to focus efforts. Where possible, activities will be embedded into existing programs to consolidate and leverage resources.
HIV Prevention Choice Manifesto
The HIV Prevention Choice Manifesto emphasizes community-led leadership and the importance of choice in HIV prevention for African women and girls. The manifesto advocates for universal access to a broad range of biomedical tools for HIV prevention and underscores the transformative potential when African women and girls lead advocacy and response efforts in HIV healthcare.
More information and background on the manifesto is available on our blog.
Px Wire May 2023
PrEP Tracker data, preparing for new products, the HIV prevention pipeline and our prevention playlist. All that and more in the latest issue of PxWire.
HIV Prevention Research & Development Investments 2021
In its 17th annual report, the Resource Tracking for HIV Prevention Research & Development Working Group documents research and development spending for the calendar year 2021. This report also analyzes funding trends spanning 22 years for the following biomedical HIV prevention options: preventive HIV vaccines, microbicides, pre-exposure prophylaxis (PrEP), treatment as prevention (TasP), voluntary medical male circumcision (VMMC), female condoms and prevention of vertical transmission (PVT). More information at hivresourcetracking.org.