Multipurpose prevention technologies (MPTs) are products designed to simultaneously address more than one sexual and reproductive health concern. This advocates’ guide shows the pipeline of products in development, discusses why MPTs are needed, investment, and what advocates can do to push for MPT development and introduction.
Advocates’ Guide to Multipurpose Prevention Technologies (MPTs)
The Lens on LEN
In 2024, Gilead Sciences released findings from the PURPOSE 1 and PURPOSE 2 trials testing lenacapavir (LEN) as HIV prevention. The PURPOSE 1 trial found 100% efficacy in preventing HIV in 5,300 cisgender women in Uganda and South Africa, and the PURPOSE 2 trial showed a 96% reduction in HIV incidence among cisgender men, trans, and non-binary individuals across multiple countries. Both trials demonstrated LEN’s safety and effectiveness in reducing HIV transmission. This advocates’ primer provides background on the product and trials; a summary of the early findings of PURPOSE 1 & 2; key questions and next steps.
SRH + HIV integration advocacy, Pandemic Accord, GPP and more!
AVAC’s round-up of resources, updates and insights this week includes a new roadmap for sexual and reproductive health (SRH) and HIV integration, resources to support an equitable Pandemic Accord, innovations in Good Participatory Practices (GPP) and more!
The power of choice in contraception, sexual health and HIV prevention this World Contraception Day
Roadmap: Sexual and Reproductive Health (SRH) Integration Roadmap

Copper Rose Zambia, as a part of CASPR and AVAC launched a new resource addressing the critical need for integrated SRH and HIV services. This roadmap provides key steps for success, focusing on collaboration, strategic mapping and targeted advocacy.
Advocate’s Guide: Advocates’ Guide to Multipurpose Prevention Technologies (MPTs)
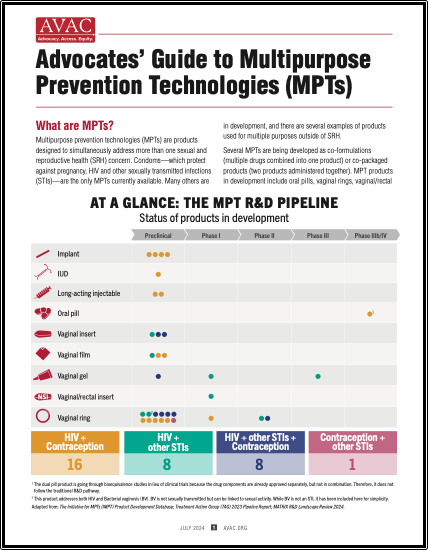
MPTs are products designed to simultaneously address more than one sexual and reproductive health concern. This advocates’ guide shows the pipeline of products in development, discusses why MPTs are needed, investment, and what advocates can do to push for MPT development and introduction.
What will it take for an equitable Pandemic Accord?
Call to Action: Pandemic Accord Priorities from the Coalition of Advocates for Global Health and Pandemic Preparedness

A group of organizations advocating for an integrated and holistic approach to preparedness that emphasizes equity, inclusion, and synergies of multiple global health programs in advancing preparedness, shares five priorities in Pandemic Accord negotiations.
UNGA Side Event: Restrategizing Civil Society Engagement for Pandemic and Global Governance

AVAC’s Sam Rick moderated CISDI’s event alongside Nina Schwalbe, Lawrence Gostin, Eloise Todd and others, reminding the audience that for pandemic prevention, preparedness and response (PPPR) to succeed, lessons from the HIV response must be integrated into the architecture being built for PPPR.
Good Participatory Practices in action
Call for Applications: Now Accepting Applications for the 2024 Good Participatory Practice Online Course
The 2024 Good Participatory Practice Online Course is now accepting applications for 30 spots! This course offering will run 14 October – 20 December 2024. The application deadline is 9 October.
Recording: Innovations in GPP

Recording / Clever Chilende Slides / Sarah Read Slides / Ntando Yola Slides
Sexual and Reproductive Health Integration Advocacy Roadmap
This roadmap addresses the critical need for integrated sexual and reproductive health (SRH) and HIV services. This integration is essential for enhancing public health outcomes, socio-economic benefits, and individual health and rights. The Roadmap aims to revitalize and sustain advocacy efforts for SRH and HIV integration, empower communities to hold stakeholders accountable for implementation, increase political and program support to enable the shift from policy to practice, and foster dynamic partnerships across research, advocacy, implementation, and policy sectors.
It was developed by Copper Rose Zambia (CRZ) as part of the Coalition to Accelerate and Support Prevention Research (CASPR). It draws on extensive input, including desk reviews, interviews, focus group discussions, and a specialized workshop held at the 2023 International Conference on AIDS and STIs in Africa (ICASA).
Avac Event
The Road Ahead – SRH Integration Advocacy
Join Copper Rose Zambia, as a part of CASPR, for a webinar, The Road Ahead – SRH Integration Advocacy. The webinar will launch the SRH Integration Advocacy Roadmap, and feature healthcare providers, advocates, and more to discuss the future of sexual and reproductive health integration.
Anna Miti Joins The Choice Agenda (TCA) as Co-Moderator
AVAC and The Choice Agenda (TCA) are delighted to welcome Anna Miti as the TCA’s new co-moderator. Based in Harare, Zimbabwe, Anna is a seasoned journalist, advocate for gender equality, an AVAC Cure Fellow, former AVAC Advocacy Fellow and co-convener of the Zimbabwe Media Science Cafe, who brings her passion for amplifying community voices to this role.
Launched by AVAC with Jim Pickett in April 2022, TCA is a global forum for advocacy on the latest in HIV prevention. With monthly webinars hosting informed discussions and a moderated listserv of nearly 3,000 subscribers from 40+ countries, TCA offers the HIV prevention community a platform to come together, learn from one another and chart the way forward. Anna will work alongside Jim and the AVAC team to foster inclusive advocacy around efforts to expand equitable access to HIV prevention tools around the world.
“As a long-time member of the TCA, I have valued it as a place for robust discussions and a vital platform to access new, timely and relevant information. I am excited to now contribute to this platform as co-moderator. Together with other advocates, I aim to strengthen the TCA’s impact and contribute even more to HIV and science advocacy.” – Anna Miti, TCA co-moderator
“On behalf of the TCA community, I am thrilled to welcome Anna into the brand-new role of co-moderator. Her dedication to HIV prevention research advocacy, her deep well of experience, and her exceptional communication skills will help us improve and expand our work to support HIV prevention research literacy and advocacy. The sun never sets on TCA, and I couldn’t be happier to have such a savvy, partner to help us take TCA to the next level.” – Jim Pickett, AVAC senior consultant and TCA moderator.
Join our Q&A with Anna, September 24
As part our webinar, Do Vaginas Demand Perfection? Implications for Event-Driven PrEP, we’ll host a 30-minute Q&A with Anna. We hope you’ll join us!

For more information about The Choice Agenda, upcoming events, or to join the listserv, visit AVAC’s The Choice Agenda page.
Brand-new PxPulse Podcast on LEN’s Impact on HIV Prevention
The promise of long-acting PrEP has been super-charged this year by studies showing the powerful efficacy of an injectable antiretroviral known as lenacapavir (LEN).
PxPulse’s new episode, Lenacapavir: The case for investing in delivering HIV prevention, goes deep on LEN. Recorded just days before Gilead’s announcement that PURPOSE 2, its second major trial of LEN as injectable PrEP, also found very high efficacy, Dr. Flavia Kiweewa, a principal investigator of PURPOSE 1, the first trial to announce efficacy, lays out the research findings and what they mean. And Chilufya Kasanda Hampongo of Zambia’s Treatment Advocacy and Literacy Campaign and Mitchell Warren of AVAC talk about how to change a long history of squandered opportunities to get rollout right.

The PURPOSE 1 trial announced findings in June that a twice-yearly injection of LEN was 100% effective among cisgender women, with zero new cases of HIV. And the PURPOSE 2 trial among cisgender men, and trans and non-binary people, was shown to reduce the risk of HIV by 96%.
LEN now enters a select category, one of five ARV-based options for PrEP that all protect against HIV if you take them. But many of the people applauding the results from PURPOSE 1 and 2 will tell you that breakthrough science like this, as hard as it is, is still the easy part. Breaking the back of the HIV epidemic demands overcoming an altogether different challenge — coordinating and accelerating every step in rolling out new products so that everyone who needs HIV prevention can get it.
Listen to this podcast to learn what must be done to finally deliver on the promise of highly effective HIV prevention, from pills to rings to injectable PrEP and beyond.
Avac Event
On the Frontlines of AMR: A Systems Approach
This is a UNGA side event at Yale Club of New York City, 50 Vanderbilt Ave, New York, NY 10017
At this year’s UN General Assembly, policymakers, advocates, diplomats, and practitioners will convene a High Level Meeting (HLM) on antimicrobial resistance (AMR). As new global commitments are made by state actors, Management Sciences for Health (MSH) will convene a group of experts to illustrate the importance of supply chains and strong health systems in combating AMR.
Through a panel discussion featuring practitioners, health workers, and advocates, MSH and partners will explore the role of supply chains, laboratory capacity, health facilities and other foundational aspects of health systems in meeting the increasingly complex challenge of AMR.
Topics discussed may include (but are not limited to): the importance of supply chains and regulatory systems in addressing AMR and supplying critical medicines, the critical role of laboratories, microbiology services and diagnostic tools, the importance of adequate water and sanitation in health facilities, and the role of healthcare workers in remaining combating and monitoring AMR.
MSH hopes that this discussion will “make real” the challenges and opportunities presented by this year’s HLM and provide real-world examples of how to address the growing threat of AMR.
Speakers:
- Opening remarks: Marian Wentworth, President and CEO, Management Sciences for Health
- Keynote introduction: Côte d’Ivoire Minister of Health Pierre Dimba
- Scene Setting Remarks: Dr. Mirfin Mpundu, Senior Antimicrobial Resistance (AMR) and Biodefense Advisor, Emerging Threats Division of the Office of Infectious Diseases, Bureau for Global Health, USAID
- Moderator: Angelo Katumba, Senior Program Manager: Partnerships & Capacity Strengthening, AVAC
- Panelist: Francis Aboagye-Nyame, Project Director, MTaPS project, Management Sciences for Health
- Panelist: Patrick Mubangizi, Africa Director, Fleming Fund, Mott MacDonald
- Panelist: Dr. Caline S. Mattar, MD, Associate Professor of Medicine and Director of Global Health
- Scholars Pathway in Internal Medicine, Washington University School of Medicine
- Panelist: Josette Vignon, Madagascar Country Director, WaterAid
- Closing Remarks: Dr. Jide Idris, MBBS, MD, MPH, Director General of the Nigeria Centre for Disease Control and Prevention

Innovations in GPP
This webinar on September 11 featured speakers from around the world with experience implementing GPP at research sites, within networks, and at the sponsorship level.
They illustrated how GPP can expand beyond the more familiar (but always reliable) CABs and town hall meetings to newer ideas like partnership-based approaches, the creation of a community scorecard, and more.
Moderator and Presenter:
- Ntando Yola, Desmond Tutu Health Foundation
Presenters:
- Sarah Read, National Institute of Allergy and Infectious Diseases
- Clever Chilende, Treatment Advocacy & Literacy Campaign (TALC)

Recording / Clever Chilende Slides / Sarah Read Slides / Ntando Yola Slides
Avac Event
Breaking New Ground: Expanding Access to Lenacapavir—Lessons from Dolutegravir and the Future of HIV Prevention
This UAN Call, titled Breaking New Ground: Expanding Access to Lenacapavir—Lessons from Dolutegravir and the Future of HIV Prevention, brought together global health experts, community advocates, and civil society organizations to discuss the challenges and opportunities in ensuring equitable access to Lenacapavir.
This webinar was hosted by Unitaid.

