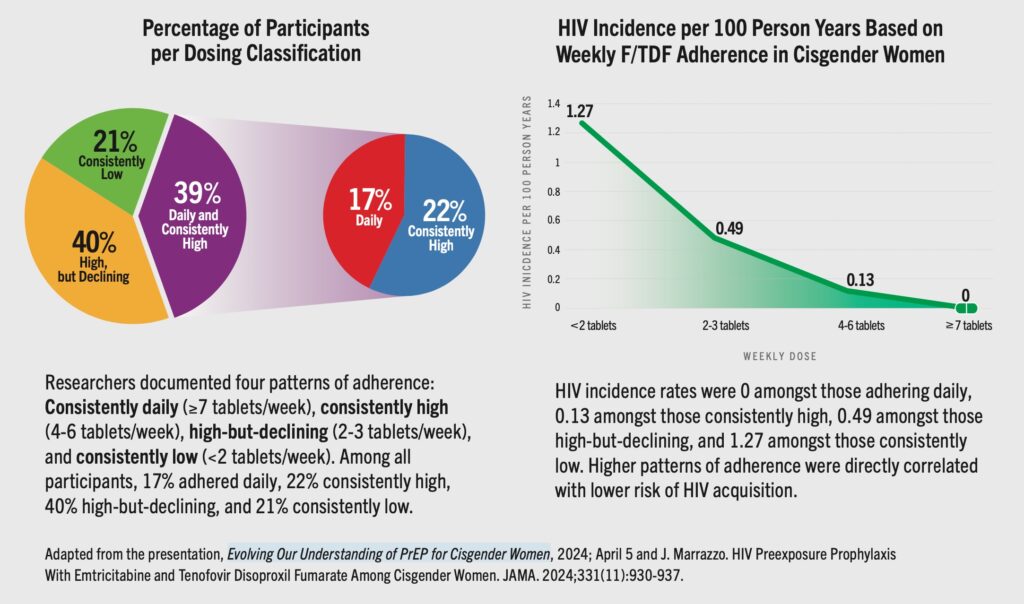
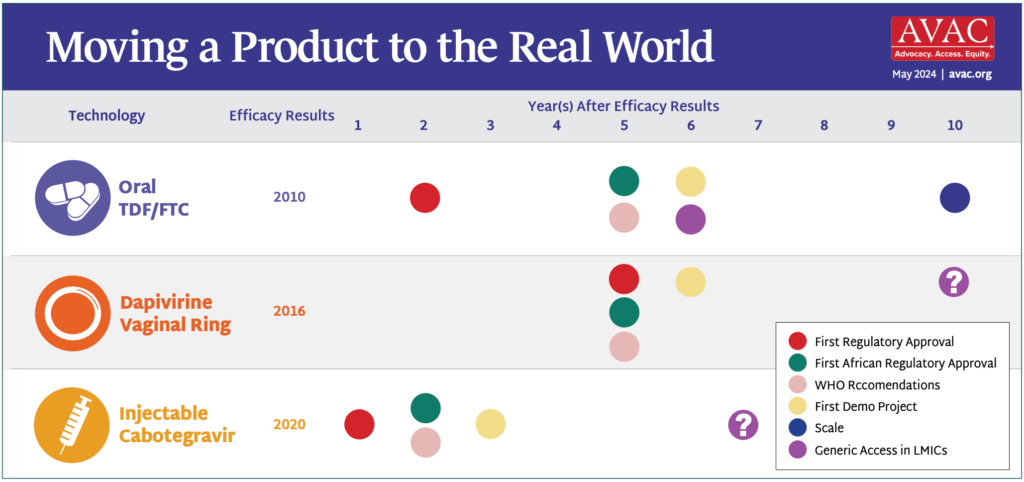
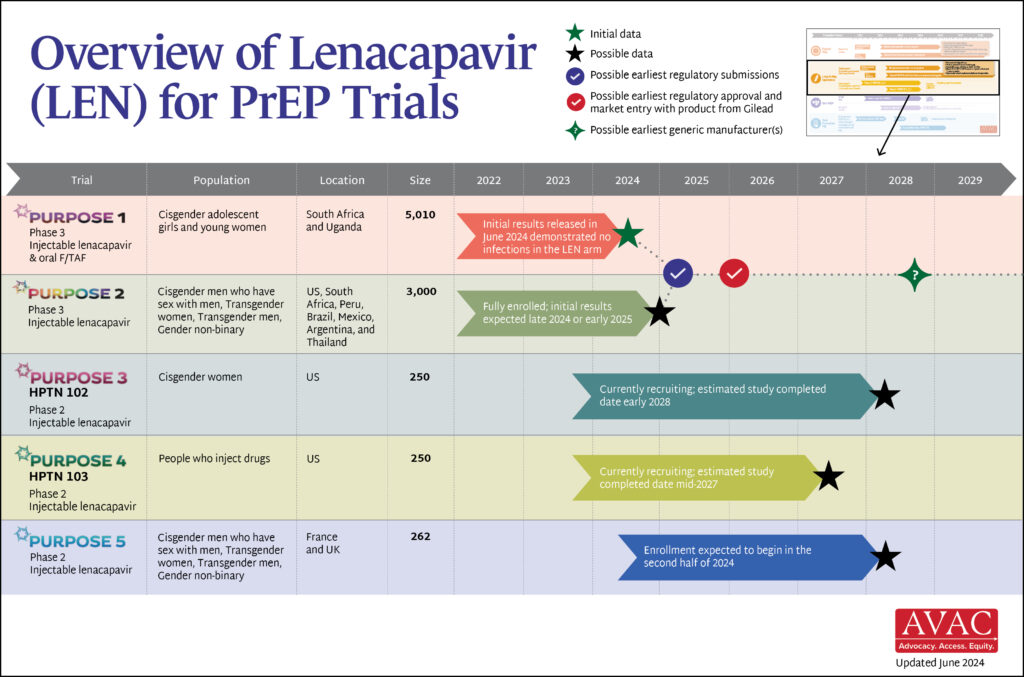
Last week’s interim result of the PURPOSE 1 HIV prevention study of injectable lenacapavir is captivating headlines. Check out AVAC’s statement here and one from the PURPOSE 1 Global Community Accountability Board and the African Women’s Prevention Community Accountability Board here. But there are LOTS of other things also happening in HIV prevention, and we’re delighted to share this roundup.
Upcoming Webinars

Responding to Project 2025’s Threats to Science, Rights and Resources
Project 2025 is part of an ongoing multi-pronged backlash to the sexual and reproductive health, gender and LGBTQ+ movements. Building on the experience of the HIV movement in fighting these same far-right forces, join this Choice Agenda webinar discussing potential responses through the lens of HIV affected communities and programs. Register here

You Get What You Measure: Why Monitoring for PrEP Choice Helps Tell Our Story
The data collected on a program determines its path and priorities. This Choice Agenda webinar will cover the current state of PrEP monitoring and evaluation, and efforts to improve and simplify data-gathering to better reflect how people use PrEP and to support choice amongst the growing array of PrEP methods. And the discussion will also focus on how data can be used to enhance the stories we tell about PrEP program implementation. Register here
Recordings and Resources
From The Lab To The Jab Webinar and Issue Briefs
Earlier this month, AVAC hosted a webinar highlighting our series of issue briefs, From The Lab To The Jab, covering research and development, mRNA technology, vaccine production, issues relevant to equitable global access to vaccines. The webinar featured panelists from the International Vaccine Institute, International Treatment Preparedness Coalition, and æqua, a think tank focused on equity and economic justice for health. Panelists discussed international initiatives for vaccine development, the current state of vaccine research and access, and how they can be improved. Read more

The GPP Body of Evidence: GPP Monitoring and Evaluation Frameworks, REAL and REAL2
GPP is an essential part of clinical trials research, and an ethical imperative to creating equitable and effective clinical trials. GPP is created by and for communities, so it looks different and takes multiple forms in different cultural contexts. This kind of responsiveness is inherent to GPP, but it also makes it difficult to measure and evaluate. In this webinar, participants will learn from the Realist Review of Community Engagement and the REAL2 review of participatory research. Each examined frameworks for evaluating community engagement efforts. We’ll also learn about the Global Health Network’s new course on evaluation, and other efforts in the field to evaluate the impact of GPP. View the recording

It’s Not Just about the Trial: GPP from discovery to delivery in TB research
GPP enhances every stage of the research lifecycle. In this webinar, our partners at TB Alliance, SMART4TB, and THINK shared experiences, lessons learned, and innovative approaches in integrating GPP at the organizational, network and situational level, from drug development through delivery. View the recording
Advocates’ Guide to Doxycycline to Prevent Bacterial STIs (DoxyPEP)
Doxycycline, an oral antibiotic, can be used as a post-exposure prophylaxis, commonly referred to as DoxyPEP, when used to prevent the acquisition of some bacterial STIs after sex. Doxycycline is inexpensive, easily tolerated, and widely available. However, questions remain regarding who will benefit most from DoxyPEP and how to implement this strategy broadly to ensure equitable access and minimize antimicrobial resistance. This guide seeks to explore and address these critical questions. Read the guide

Episode 3: The Promising Science
Our Mitchell Warren speaks to ViiV’s Kimberly Smith in this episode of the Foreign Policy podcast series ‘can we end epidemics?’ about the future of HIV science and the challenges we need to overcome on our journey to finding a cure. Listen
We hope these conversations and resources are helpful in your advocacy. Stay tuned for our upcoming advocates’ primer on lenacapavir and our roadmap to the AIDS 2024 conference in Munich.