Between the period January-September 2024 to January-September 2025, PrEP initiations fell between 13% and 66% in selected high-volume PrEP countries where ministries of health (MoH) were able to provide data. Among the five countries depicted, four saw significant decline in PrEP uptake. All four relied on PEPFAR PrEP programs and were disrupted by stop work orders (SWO).
Change in PrEP Initiations from 2024 to 2025 in Five Countries
Global Health Watch: Reflecting on 1 Year of Chaos and What’s Next, Congress Pushes Back, the Future of WHO, US Undermines African Authority
This week marks 365 days of disruption and chaos across global health, with many organizations and journalists reflecting on one year after the foreign aid freeze and what the future of global health looks like. This week also saw the US Congress beginning to reassert its role on health funding, ongoing US disengagement from WHO, and new reporting on the hepatitis B vaccine trial in Guinea-Bissau.
We are also watching closely for news of an expanded Global Gag Rule, that is reportedly coming out later today, just as this issue goes to press. It is anticipated that this new gag will include a dramatic expansion beyond abortion and now underscore the US administration’s war on gender, diversity, equity and inclusion. We’ll cover this in next week’s Global Health Watch; the struggle, most definitely, continues.
365 Days of Chaos and Disruption, What Comes Next for Global Health
This week marks one year since the US Presidential administration issued a deeply cruel executive order freezing foreign assistance, halting billions of dollars in already-approved funding under the bad-faith claim of a “90-day review”. What followed was not a brief pause, but a drawn-out, chaotic disruption that stopped life-saving work across the globe, shutting down valuable organizations, and harming lives, health, and livelihoods. And that was just the beginning of a year of chaos. Many organizations and media coverage noted a growing recognition that the past year’s disruptions to global health were not just destructive, but catalytic, forcing a reckoning with how systems are built and for whom. Reporting from Bhekisisa reflected on how the sudden halt in US HIV funding exposed deep vulnerabilities in over-reliance on external aid, while also accelerating conversations about domestic financing, integration, and sustainability in the HIV response moving into 2026. At the same time, analyses from Health Policy Watch, Science, Nature, and The Lancet laid bare the human and scientific costs of abrupt policy shifts, shuttered programs, fractured research networks, and lost trust, while underscoring that simply restoring old funding streams will not be enough to meet future challenges.
IMPLICATIONS: Together, these stories and analyses point to the need to rethink and rebuild with stronger country ownership, diversified and predictable financing, resilient research institutions, and governance structures that prioritize equity, accountability, and community leadership. Rather than recreating the same architecture, reimagined global health systems must be less dependent on external donors and political ideology. As Global Health Watch has tracked for 52 weeks, the path forward is not about returning to the pre-foreign aid freeze status quo, but about using this period of disruption to construct a more durable, equitable, and responsive global health architecture. As Canadian Prime Minister, Mark Carney, said so clearly at this week’s World Economic Forum in Davos, Switzerland, “Nostalgia is not a strategy.”
READ:
- Fighting for billions: The legal battle to keep US foreign aid alive—Devex
- [PODCAST] One year after Trump: The day HIV funding changed forever — and what came next—Bhekisisa
- US science after a year of Trump—Nature
- One Year Later: The Effect of US ‘Chainsaw’ on Global Health—Health Policy Watch
- Four paradigm shifts to shape an agenda for global health reforms—The Lancet
- Damage assessment: Which of Donald Trump’s changes are likely to last—and which will fade?—Science
Congress Steps Up In Defending Domestic and Global Health
The US Congress is beginning to reassert its constitutional role in shaping federal spending. The joint House and Senate FY26 funding bill released last week covering foreign operations would maintain funding for core global health priorities, including HIV, tuberculosis, malaria, polio, family planning and reproductive health, neglected tropical diseases, Gavi, and UN agencies such as UNAIDS, UNICEF, and UNFPA. This week, legislators rejected nearly $2 billion in proposed cuts to US domestic HIV and related programs through the Labor, Health and Human Services Education and Related Appropriations Act (LHHS), a “major victory” credited to sustained advocacy by people living with HIV, advocates, and service providers. The proposed bill largely preserves funding for domestic public health and biomedical research. As AVAC’s Suraj Madoori said in a statement, “These crucial wins for global and domestic HIV now require us to not hold back, and urge Congress to swiftly approve all the FY26 bills, push the President to sign them, and for us to ensure accountability in the administration to spend and implement this lifesaving funding as instructed by the people and those who represent us in Washington D.C.”
IMPLICATIONS: Together, these bills signal a return to a bipartisan appropriations process and, if enacted, provide a basis to push back against unilateral cuts by the administration. They also signal bipartisan pushback against attempts to cut health and scientific research investments. By rejecting the steep cuts proposed by the administration, they stabilize lifesaving programs and protect the research enterprise. The House passed the bills on Thursday, and the focus now shifts to the Senate, which must pass the bills by January 30. Then onto the President to sign, and, most critically, for the administration to actually spend all Congressionally appropriated funds.
READ:
- MAJOR VICTORY: Bipartisan FY26 Bills Reject Nearly $2B in Proposed HIV Funding Cuts—Save HIV Funding
- Beyond the Numbers: Three Policy Shifts in the FY26 Funding Bill Quietly Reshaping U.S. Global Health—Lights, Camera, Equity Substack
- Limit on multiyear funding of NIH grants is a sticking point in Senate budget talks—STAT
US Disengagement and the Future of WHO
This week also marks one year since the US announced its intended departure from World Health Organization (WHO). Debate over its future and the US’ role within it intensified as pressure from parts of the administration collided with growing concern over the consequences of disengagement. US officials and policymakers aligned with the “America First” strategy call for WHO to be fundamentally reformed or replaced. Analysts note that the US remains a formal member of WHO until debts are paid. Meanwhile, the US administration continues to withhold funding and delay payments, leaving millions of dollars in unpaid US obligations.
IMPLICATIONS: The current state, where the US remains technically engaged but substantively absent, poses serious risks for global health governance, disease surveillance and pandemic preparedness. As analyses from CSIS underscores, WHO reform is both necessary and possible, but meaningful reform requires constructive engagement, predictable financing, and political leadership, not abandonment.
READ:
- MAGA-backed researchers call for WHO to be ‘reformed or replaced’ on eve of US withdrawal—The Telegraph
- As U.S. prepares to exit WHO, it is stiffing the agency on a large bill—STAT
- The Future of the WHO—and How the United States Can Shape It—CSIS
- A US return to the World Health Organization could hinge on whether Trump approves of its next leader—Politico
Hepatitis B Vaccine Trial in Guinea-Bissau Undermines African Authority
Reporting this week is exposing a pattern of US actions that show deep disregard for African public health leadership, ethical research standards, and sovereignty. The US-funded hepatitis B vaccine trial in Guinea-Bissau is at the center of this. As we reported in previous weeks, the unethical trial would delay birth-dose vaccination for thousands of newborns despite overwhelming evidence, and WHO guidance, that immediate vaccination saves lives. According to Rolling Stone, the study was championed by US officials aligned with anti-vaccine ideology and advanced even as Africa CDC officials raised alarm and indicated the trial should be halted. At the same time, the US administration has been marginalizing Africa CDC more broadly, cutting engagement, undermining its authority, and sidelining African institutions in favor of unilateral decision-making. This is being reinforced, as The Guardian reports, by members of the US administration urging US diplomats to emphasize American “generosity” to African leaders even as USAID programs are shuttered and health funding withdrawn.
IMPLICATIONS: Together, these developments signal a dangerous erosion of respect for African expertise, autonomy, and ethical authority in global health. Pushing forward research designs that would withhold proven interventions—while dismissing objections from Africa CDC and African scientists—revives patterns of extractive and unethical research long condemned by the global health community. Undermining Africa CDC while advancing ethically dubious trials weakens trust, damages partnerships, and threatens progress against hepatitis B, HIV, and other diseases where Africa has led with scientific excellence.
READ:
- Trump administration to block aid from subsizing DEI and trans rights overseas—The Guardian
- Trump’s HHS Trashes Top African Health Organization as “Fake” and “Powerless”—Futurism
- Head of US Africa bureau urges staff to highlight US ‘generosity’ despite aid cuts—The Guardian
- HHS Gave a $1.6 Million Grant to a Controversial Vaccine Study. These Emails Show How That Happened—Rolling Stone
- Lipstick on a Pig: Why the Amended Hepatitis B Birth-Dose Trial in Guinea Bissau Remains Ethically Indefensible—BK’s Substack
- ‘Suspended or Cancelled’: Guinea-Bissau Health Minister Halts Controversial Hepatitis B Trial—Health Policy Watch

Read AVAC’s ‘One Year Later’ series
This five-part series reflects on the tumultuous events of the past 365 days across five global health issues: the erosion of US foreign aid; dismantling of the research enterprise; attacks on vaccine policy; and shifts in the global health architecture.
What We’re Reading
- US cuts to HIV programs in sub-Saharan Africa pose global risk, experts say—CIDRAP
- Trump dismantled USAID. Now these aid workers are running for office—Devex
- EU Parliament Backs Critical Medicines Act, Sparking Supply Concerns In Africa—Health Policy Watch
- A big announcement on AI in Africa—Bill Gates
- Multilateral paralysis is harming global health. Gavi’s ‘minilateralism’ can get us back on track—World Economic Forum
- Trump one year on: How six US researchers plan to protect science amid chaos and cuts—Nature
- Talking back: An unprecedented assault has forced the U.S. scientific community to rethink its advocacy tactics—Science
- The little-known vaccine panel that could have big consequences—STAT
- The near death – and last-minute reprieve — of a trial for an HIV vaccine—NPR
- When Trump Took a Whack at the C.D.C., Atlanta Lost Something, Too—New York Times
- What Does America’s Government to Government Collaboration for Health Look Like?—Emily Bass Substack
- Is the $364 million US-Lesotho Health MoU unconstitutional?—Newsday
- Africa pushes back on US health deals over data, power—SciDevNet
- Judge Orders Trump Administration To Restore $12M to American Academy of Pediatrics—The Hill
- Most Vaccine Hesitancy Can Be Successfully Overcome, New Lancet Study Finds—Health Policy Watch
- Pazdur warns that politics, ‘chaos’ are damaging FDA—STAT
- Gates Foundation unveils $9 billion budget and plans to cut staff—Associated Press

As new IDSA CEO, Jeanne Marrazzo warned in a webinar this week, rising HIV infections threaten progress, but “this is not a time to despair. It’s a time to fight.” Watch the recording and explore the 2025 People’s Research Agenda, which tracks the science, flags gaps, and centers community priorities to keep HIV prevention moving forward.
Resources
One Year Later
“The last year has been one of chaos, anger, panic, and frustration. I think the coming year has to be one of strategic rebuilding and building something different—not building back but building forward. That means a different architecture at the country level, community level, regional level, and global level. A year from now, I suspect we’ll see fewer acronyms, but hopefully the ones that remain will be stronger than ever.” —AVAC’s Mitchell Warren, Bhekisisa podcast
This week marks one year since the US Presidential Administration issued a deeply cruel executive order freezing foreign assistance, halting billions of dollars in already-approved funding under the bad-faith claim of a “90-day review”. What followed was not a brief pause, but a drawn-out, chaotic disruption that stopped life-saving work across the globe, shutting down valuable organizations, and harming lives, health, and livelihoods. And that was just the beginning of a year of chaos.

To mark this moment, AVAC’s One Year Later series reflects on the impact of the past 365 days on five key areas of global health and development:
- The erosion of US foreign aid
- The assault on vaccine science and policy
- The dismantling of the research enterprise
- The cruel irony of funding cuts in the context of the breakthrough technology of long-acting lenacapavir for PrEP
- The profound shifts underway in global health architecture
These pieces show how the field navigated a year defined by disruption and resilience—and how policy decisions reverberate through science, programs, and communities.
The year has been a profoundly transformative one for AVAC. The Devex in-depth retrospective, Fighting for Billions: The legal battle to keep US foreign aid alive, chronicles the ongoing lawsuits brought by AVAC, the Global Health Council and partners challenging the foreign aid freeze. The piece highlights how legal action became a critical line of defense against the dismantling of lifesaving programs and why the outcome still matters.
“For me, the best message I can say is a year later, we as a community are still standing. And that is a resilience in its own right. We are going to succeed in global health and development. Not because of what happened in the last year, but in spite of it.” —AVAC’s executive director Mitchell Warren on Bhekisisa’s new podcast, One year after Trump: The day HIV funding changed forever—and what came next
As Jeanne Marrazzo, the new CEO of the Infectious Diseases Society of America (IDSA) shared on our webinar earlier this week showcasing the 2025 update of the People’s Research Agenda (PRA), “We cannot yell it from the rooftops loud enough that new infections are going to rise and undermine efforts to end AIDS as a public health threat. But this is not a time to despair. It’s a time to fight. It’s a time to dig in and recognize not just what we’ve accomplished and why we need to protect that, but why we need to continue to move forward.” See the recording here and learn more about the PRA, which tracks the science, highlights where investments align—or fail to align—with community priorities and identifies critical gaps that must be addressed to ensure the prevention pipeline meets the needs of diverse populations.
The past year has reshaped global health—and AVAC—in ways that will be felt for years to come. The events of the past year also show that advocacy, evidence, and community leadership matter. Some courts provided the necessary check on power; advocates rallied; and scientists and civil society raised their voices and documented what was lost—and what must be protected (see 24 Hours to Save AIDS Research).
With your support, AVAC’s weekly Global Health Watch newsletter, now in its 52nd week, continues to track what happens, elevate what’s at risk, and help all of us navigate what comes next. Thank you to our community which stops at nothing to safeguard hard-won progress against HIV and in advancing global health equity.
An “Innovation Pile-Up” in Next-Generation LA-PrEP is Possible
The HIV prevention market is headed toward a period of significant opportunity—and possible congestion—as a slate of new products are on track for continued development and potential introduction to the market in 2027 and 2028. Markets and policies must be built to support the products in the market already, so that new options can be rapidly deployed and deliver impact. Otherwise, the field will squander time and money, with epidemic control slipping further out of reach.
Dapivirine Vaginal Ring Regulatory Approval
Regulatory approvals, pending decisions, and appeals as of January 2026. For product approvals, volumes, implementation, and price comparisons of long-acting PrEP, visit our dashboard on PrEPWatch.org.
HIV Prevention Product Overview
The graphic provides an overview of PrEP products currently available and in late-stage clinical trials.
Cabotegravir Regulatory Approval
Regulatory approvals and those pending for cabotegravir as of January 2026. For product approvals, volumes, implementation, and price comparisons of long-acting PrEP, visit our dashboard on PrEPWatch.org.
Global Health Watch: A Year That Reshaped Global Health
The Lancet journal ended the year with a provocative editorial – 2025: an annus horribilis for health in the USA. But sadly, it was not just in the US; it has been a year of chaos and disruption globally. This 49th issue of Global Health Watch looks back—like many news stories this week—across 2025 to highlight the most consequential decisions, disruptions, and debates that defined the year and will continue to shape what comes next.
The Foreign Aid Freeze and the Legal Fight to Restore it
On the first day in office, the new US Administration issued a sweeping foreign aid freeze that halted life-saving global health and HIV programs, severed active grants, research underway and cost millions of people their lives and livelihoods. In less than a month, AVAC responded suing the President, the State Department and the US Agency for International Development (USAID). The Global Health Council also led a similar lawsuit challenging the freeze as unlawful and harmful. Together, the two cases argued for months in various courts that the foreign aid freeze not only jeopardized health as a human right but also bypassed congressional authority and undermined trust in US leadership. Ultimately, the cases unlocked millions of dollars of development assistance for work done in January and February, but millions more dollars expired at the end of the fiscal year in September. The cases are ongoing and as important as ever, both to restore foreign assistance and to re-assert that it is Congress (and not the President) who has the power of the purse.
READ:
- The Monthslong Legal Battle to Save Foreign Aid—New York Times
- AVAC v. United States Department of State—AVAC
- “Lift the Freeze”: HIV/AIDS Advocates in Fight over Trump Foreign Aid Cuts—Democracy Now
- The Supreme Court’s Major Cases During the 2025-2026 Term—Washington Post
- Supreme Court has expanded presidential powers under Trump. How far will it go?—Washington Post
Research Under Assault
Science faced underfunding and systematic destabilization in 2025. In just one month under the new US Administration, the National Institutes of Health (NIH) abruptly canceled approximately 1,800 research grants. By April, mass layoffs and forced reassignments across Health and Human Services (HHS) agencies, including the Centers for Disease Control and Prevention (CDC), NIH, and US Food and Drug Administration (FDA), further crippled each agency’s capacity and expertise. A proposal to drastically cut the overall NIH budget and consolidate its 27 institutes was soon introduced along with the fiscal year 2026 budget, which proposed an $18 billion cut from the NIH and $1.5B cut in HIV prevention. Around the same time, the NIH signaled a major shift away from investments in basic science and clinical research, undermining the discovery pipeline that fuels future breakthroughs. Then, in November, HHS ordered the CDC to phase out all “non-essential” nonhuman primate research, threatening foundational preclinical studies, including those that have been pivotal to HIV PrEP and PEP, amongst many other health priorities. These actions were compounded by a pause or effective ban on some international research collaborations, a proposed cap on indirect cost rates that support core university infrastructure, and changes to the scientific review processes, together weakening the systems that sustain rigorous, independent research.
READ:
- The Trump Administration’s Most Paralyzing Blow to Science—The Atlantic
- HIV Prevention R&D at Risk: Tracking the Impact of US Funding Cuts—AVAC
- Trump has blown a massive hole in global health funding—and no one can fill it—Science
- Trump administration agrees to reconsider frozen and denied NIH grant submissions related to DEI—STAT
- Presidential HIV council warns proposed cuts could reverse decades of progress—CNN
- Nearly 2,000 top researchers call on Trump administration to halt ‘assault’ on science—STAT
The Cruel Irony of the Best Shot at HIV Prevention
Despite all the chaos, 2025 offered remarkable milestones in HIV prevention science, and a stark illustration of the contradictions shaping global health. Injectable lenacapavir for PrEP (LEN), the six-month injectable, which provides nearly complete protection against HIV infection, moved with unprecedented speed from regulatory approvals and guidelines to real-world introduction. South Africa and Zambia authorized LEN within months of US and EU regulatory approvals; the World Health Organization (WHO) rapidly issued guidance and prequalification; and initial LEN delivery began in Brazil, Eswatini, South Africa, and Zambia, setting the stage for expanded access in 2026. At the same time, efficacy trials began of the next promising PrEP option, the monthly oral candidate MK-8527, reinforcing what’s possible when innovation, evidence, and advocacy align.
Yet, all this scientific momentum occurred alongside the deepest assault on global health and the systems that make it possible. The cruel irony of this moment is that as the science breaks barriers, the infrastructure meant to support discovery, evaluation, and equitable delivery is being weakened, threatening the very gains the field has fought decades to achieve. As AVAC has emphasized, the greatest opportunity in HIV prevention lies in speed, scale, and equity.
READ:
- In under a year, Trump administration has threatened decades of progress in global fight against HIV/AIDS—Prism
- A ‘Breakthrough’ Drug to Prevent HIV, an ‘Unprecedented’ Rollout—NPR
- When Politics Trumps Science: Why the US isn’t giving South Africa LEN—Bhekisisa
- ‘Best of Times, Worst of Times for HIV Prevention’—Managed Healthcare Executive
Attack on Vaccine Science
Actions in the last 11 months have eroded evidence-based policy, disrupted institutional capacities, and deepened mistrust and uncertainty in vaccine science. In May, NIH’s National Institute of Allergy and Infectious Diseases (NIAID) announced that funding for the Consortia for HIV/AIDS Vaccine Development (CHAVD) would end after the current grant cycle in June 2026 — eliminating $67 million annually and about 10% of global HIV vaccine research funding. Then, $500 million in Biomedical Advanced Research Development Authority (BARDA) grants for research and development of the mRNA vaccine platform were soon cancelled, and members of the CDC’s Advisory Committee on Immunization Practices (ACIP) were replaced. The US also stopped supporting Gavi, the vaccine alliance, and language on the CDC website was replaced with anti-science and anti-vaccine sentiment. As AVAC said in an August statement, “These actions dangerously sow vaccine disinformation and mistrust, which has proliferated since the COVID-19 pandemic. Dangerous ideology results in dangerous policymaking, putting many lives at stake and complicating efforts to both discover and implement clinical and cost-effective interventions to make America and the world healthier, safer, and more prosperous.”
READ:
- We Don’t Seem to Be Making America Healthy Again—New York Times
- This Is the Damage Kennedy Has Done in Less Than a Year—New York Times
- Experts Question Denmark’s Vaccine Program as a Model for the U.S.—New York Times
- The U.S. vaccine schedule is a jet engine. Denmark’s is a toy plane.—Washington Post
- The Guardian view on mRNA vaccines: they are the future – with or without Donald Trump—The Guardian
Changing Global Health Architecture
As rising nationalism, geopolitical tensions, and funding retrenchment intensify, the architecture of global health and how countries engage in it and with one another is being fundamentally reshaped. Longstanding multilateral systems are giving way to a more fragmented, country-to-country model under the US America First Global Health Strategy. The strategy prioritizes bilateral health Memorandums of Understanding (MoUs) with individual countries in exchange for funding support, data sharing, and pathogen access, signaling a major recalibration away from traditional multilateral institutions and frameworks. Meanwhile, the US stepped back from longstanding global health platforms including an unprecedented absence at the World Health Assembly, withdrawal from the WHO, and diminishing support for joint initiatives like Gavi, the vaccine alliance. Civil society and advocates are actively debating what this means for shared goals and equity in global health, even as institutions like WHO and UNAIDS explore how to adapt in a rapidly evolving landscape.
READ:
- ‘America First’ in Global Health: Oxymoron or opportunity?—Devex
- 4 more African nations secure $2.3bn US health funding under America First strategy—Business Insider Africa
- Writing in Real Time: How 2025 Rewired Global Health—and My Work Alongside It—Lights, Camera, Equity! Substack
- UNAIDS board launches new process for transition amid sunset calls—Devex
What We’re Reading:
- In a tumultuous year, US health policy has been dramatically reshaped under RFK Jr.—Associated Press
- Podcast: Unmasking Global Health: Reflections on 2025—Global Health Unfiltered
- How Cameroon Fought to Save Its Malaria Program After the U.S. Cut Critical Funding—New York Times
- Evaluating the impact of two decades of USAID interventions and projecting the effects of defunding on mortality up to 2030: a retrospective impact evaluation and forecasting analysis—The Lancet
- Podcast: Global Health | The rollercoaster continues in 2026—Pandemia (German language)
Global Health Watch: UNAIDS launches review, NIH GoF controversy, support for IDSA’s Jeanne Marrazzo, UK authorizes LEN for PrEP
This week the UNAIDS board approved a new Global AIDS Strategy and launched a formal review of the agency’s future; turmoil at the NIH continues over gain-of-function research; and the scientific community rallies around the Infectious Diseases Society of America’s (IDSA) appointment of Jeanne Marrazzo as its chief executive officer. Also, the UK regulatory agency approved lenacapavir for PrEP (LEN) marking its seventh regulatory approval in just six months.
UNAIDS Launches Review Process on its Future
Following last week’s intense discussions at the UNAIDS Programme Coordinating Board (PCB), the UNAIDS board this week launched a new, formal process to examine the organization’s future and potential transition pathways. This comes from within the UN80 reform initiative that proposed to sunset UNAIDS by the end of 2026. But civil society and PCB members pushed back, and the board agreed to initiate a “structured review” that explores different scenarios for UNAIDS’ role, mandate, and positioning within a changing global health architecture. This announcement came on the heels of the PCB approving the Global AIDS Strategy for 2026–2031 and alarms raised by civil society about funding cuts, service disruptions, and the risk of losing a central coordinating body at a critical moment in the HIV response.
IMPLICATIONS: The launch of this process to examine UNAIDS’ future raises important questions about governance, accountability, and continuity in the global HIV response. Civil society’s strong pushback underscores that any reform must preserve UNAIDS’ core mandate and ensure that the global HIV response remains centered on those most affected — especially women, girls, and key populations — rather than being quietly dismantled at a moment of crisis.
READ:
- UNAIDS board launches new process for transition amid sunset calls—Devex
- New Global AIDS Strategy and Transition Working Group adopted at UNAIDS’ 57th Board meeting—UNAIDS
Continued Turmoil at the NIH – Gain-of-Function (GOF) Research
Turmoil at the NIH continued this week as, John Beigel, a prominent influenza researcher and acting director of NIAID’s Division of Microbiology and Infectious Diseases (DMID), resigned following controversy over an NIH-supported seasonal flu virus study and how its potential risks were assessed and communicated. Beigel’s departure unfolds amid ongoing debate over how the NIH defines and oversees gain-of-function (GOF) research—work that could increase the transmissibility or virulence of pathogens with pandemic potential.
Science reports that the controversy was a “‘pseudomanufactured concern’ that was meant to force him out, so officials could bring in a researcher who has strongly supported Trump.” Beigel is being replaced by an infectious disease scientist from NIH’s Fogarty International Center and who has publicly expressed support for the President and donated to his affiliated political committees.
IMPLICATIONS: Alongside last week’s revelations and Jeanne Marrazzo’s whistleblower lawsuit, Beigel’s departure heightens concerns about instability and governance at NIH at a time when scientific leadership and public trust are critical. Debates over GOF research, including its definition, oversight, and whether the White House or the NIH sets the rules show the precariousness of the agency. As Science reports, concerns about GOF work have gained momentum with the popularization of the belief that Chinese scientists who received NIH funding created the virus that caused the COVID-19 pandemic. Many Republicans have promoted this unproven theory, and Trump signed an executive order in May that called for stricter oversight of GOF work.
READ:
- NIH official resigns after flap over risks of seasonal flu virus study—Science
- Researchers have a moral obligation to push back when their studies are twisted to promote false health claims—STAT
Leaders Support Jeanne Marrazzo as new CEO of the Infectious Diseases Society of America (IDSA)
Leaders in the scientific and infectious disease communities praised the Infectious Diseases Society of America (IDSA) appointment of Jeanne Marrazzo as its next chief executive officer. Former NIAID Director Anthony Fauci called her a “superb choice,” and AVAC’s Mitchell Warren said, “It speaks to IDSA’s desire to emphasize science over politics and science over ideology, and that’s what you will get with Jeanne Marrazzo.” Virologist Angela Rasmussen, said Marrazzo’s appointment “suggests to everybody who’s a member of that professional society that they’ve got a leader who’s actually going to do something about this rather than trying to protect the institution more than its members.” Marrazzo begins her tenure January 12.
READ:
- As Marrazzo prepares for helm at IDSA, scientific community praises choice—Center for Infectious Disease Research and Policy
UK’s Medicines and Healthcare products Regulatory Agency (MHRA) Approves LEN for PrEP
This is the seventh regulatory approval of LEN for PrEP. See AVAC’s detailed map of regulatory approvals, pending decisions, and appeals, along with other LEN resources here.
What We’re Reading
- Trump removes nearly 30 career diplomats from ambassadorial positions—Associated Press
- 2025: an annus horribilis for health in the USA—The Lancet
- Some patients face hurdles getting HIV prevention drugs. Here’s what to know—NPR
- Podcast: Trans Life: How to Survive Trump’s 2nd Administration | Dr. Tatyana Moaton—A Shot in the Arm
- Kennedy ‘deeply committed to ending animal experimentation’—Science
- Perseverance Is the Prescription for Global Health Challenges—Harvard
- I’m the former head of Pfizer R&D. I’m very worried about biopharma’s future—STAT
- America’s new top health diplomat has strong opinions on abortion and gender—NPR
- The vaccine cold chain: A fragile link in global health infrastructure—Health Policy Watch
- UNICEF, Gavi deal slashes malaria vaccine price to $2.99 per dose—Devex
- How HIV Capsid Research Sparked a New Era in Prevention—American Society for Microbiology
- U.S. Global Health Country-Level Funding Tracker—KFF
- Forget quiet quitting, the State Department appears to be quiet hiring—Devex
- Trump Officials Celebrated With Cake After Slashing Aid. Then People Died of Cholera.—ProPublica
- Exclusive: Senate Democrats introduce bill to protect UN Population Fund—Devex
- California Hires Former C.D.C. Officials Who Criticized Trump Administration—New York Times
- Update on Lives Lost from USAID Cuts—CGD
- U.S. Global Health Country-Level Funding Tracker—KFF
30 Years of Standing for Science and Equity
This month, AVAC marked our 30th anniversary. Over three decades, the HIV field has evolved dramatically—but what we do, and why we do it, has remained constant: standing for science, equity, and community leadership, and ensuring evidence drives decisions that affect people’s lives. We’ve been able to do this work because of your partnership and support, and we are deeply grateful.
Last week, we also released the 2025 update of the People’s Research Agenda (PRA), which tracks the science, highlights where investments align—or fail to align—with community priorities, and identifies critical gaps that must be addressed to ensure the prevention pipeline meets the needs of diverse populations. After ten months of disruption and uncertainty across biomedical research and global health, we hope this agenda helps share a path forward, one that will demand sharper priorities, smarter investments, and a balanced portfolio focused on real epidemic impact.

At the same time, we are seeing real progress. In just the past month, people in Brazil, Eswatini, South Africa, and Zambia began receiving the first doses of lenacapavir for PrEP (LEN) through early implementation programs outside the US, with additional deliveries of LEN planned for Eswatini, Zambia, Kenya, Lesotho, Mozambique, Nigeria, Uganda, and Zimbabwe.
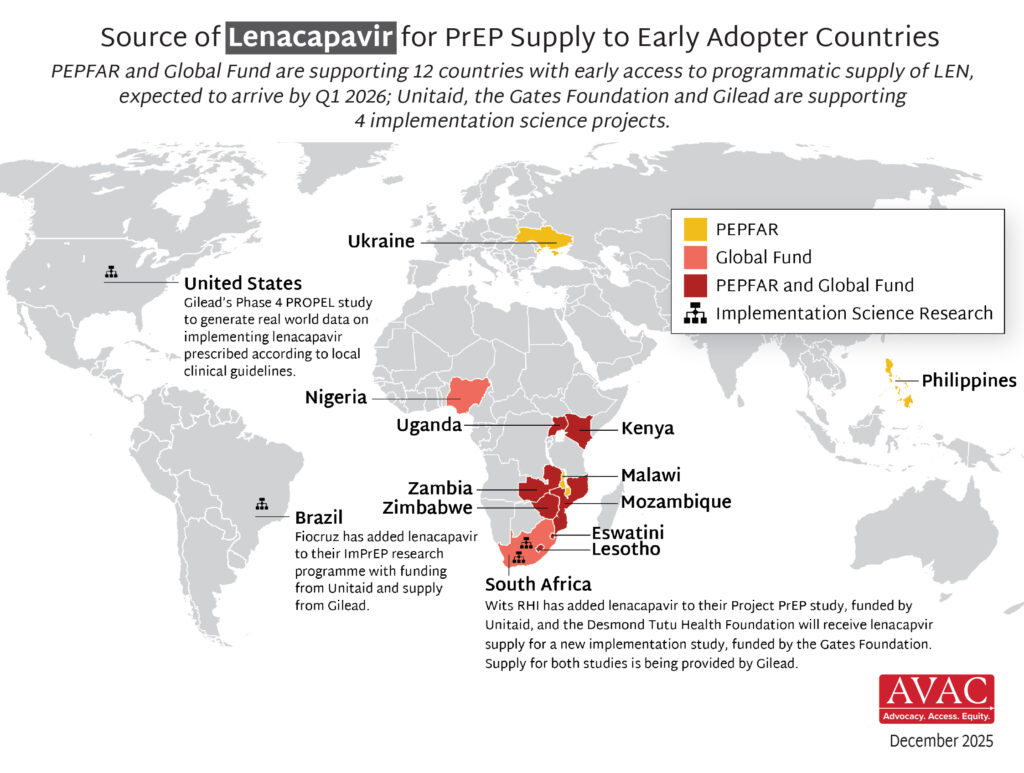
AVAC’s updated map of Global Fund and PEPFAR-supported LEN supply shows how quickly this breakthrough is moving and what’s possible when political will, funding, community engagement, and innovation align. But there is still so much more to do – as we wrote last week, science alone won’t get us there: the future of HIV prevention depends on speed, scale and equity.
As these advances continue to develop, AVAC will continue to help make sense of the rapidly shifting global health landscape. From World AIDS Day passing with little acknowledgment by the US government, to the LEN rollout (and South Africa being left behind), to the gutting of foreign aid and impact on HIV prevention and global health, to new bilateral health MoUs under the US “America First” strategy, AVAC has shared real-time analysis and context on the most pressing issues of December. Global Health Watch, now in its 46th week, will continue providing consistent, trusted context so you can navigate the turmoil with clarity, purpose and solidarity.
As we enter our fourth decade, your support makes it possible for AVAC to keep tracking the science, elevating community priorities, and delivering real-time analysis when it matters most. If you’re able, we invite you to consider making a year-end gift to sustain this work.
Thank you for being part of this work, and for standing with AVAC.