May 18th is recognized as HIV Vaccine Awareness Day (HVAD), and this year it is a time of deep reflection and potential. Three major HIV vaccine trials have ended in no efficacy since 2020, but the field knows more than ever that a vaccine is still needed for a durable and sustainable end to the pandemic – and has new insights into possible vaccine strategies that might one day effectively protect against HIV.
This year, AVAC and CASPR partners are casting a spotlight on the many issues and opportunities for HIV vaccine science. See below for three important conversations
Podcast: Listen to AVAC’s PxPulse podcast, Evolving Strategies for an HIV Vaccine: One researcher explains where the field is going and why, featuring Katy Stephenson of Harvard Medical School, Beth Israel Deaconess Medical Center, and the Center for Virology and Vaccine Research. In conversation with AVAC’s Jeanne Baron, Katy provides an accessible breakdown on the status of the field of HIV vaccine research, including details on recent trial results that proved ineffective and what’s next (and exciting) in HIV vaccine advocacy and research.
“The field needs to expand the diversity of scientists who are thinking of new ideas. We haven’t gotten far with the ideas coming out exclusively from the United States and Europe. We need to bring in young scientists of diverse backgrounds all over the world to think of ideas we can’t even imagine.”
“We now know that non-broadly neutralizing antibodies don’t work, but broadly neutralizing antibodies can work. It’s a big milestone in the field to have that kind of knowledge now.”
Katy Stephenson, Harvard Medical School and Beth Israel Deaconess Medical Center, and the Center for Virology and Vaccine Research
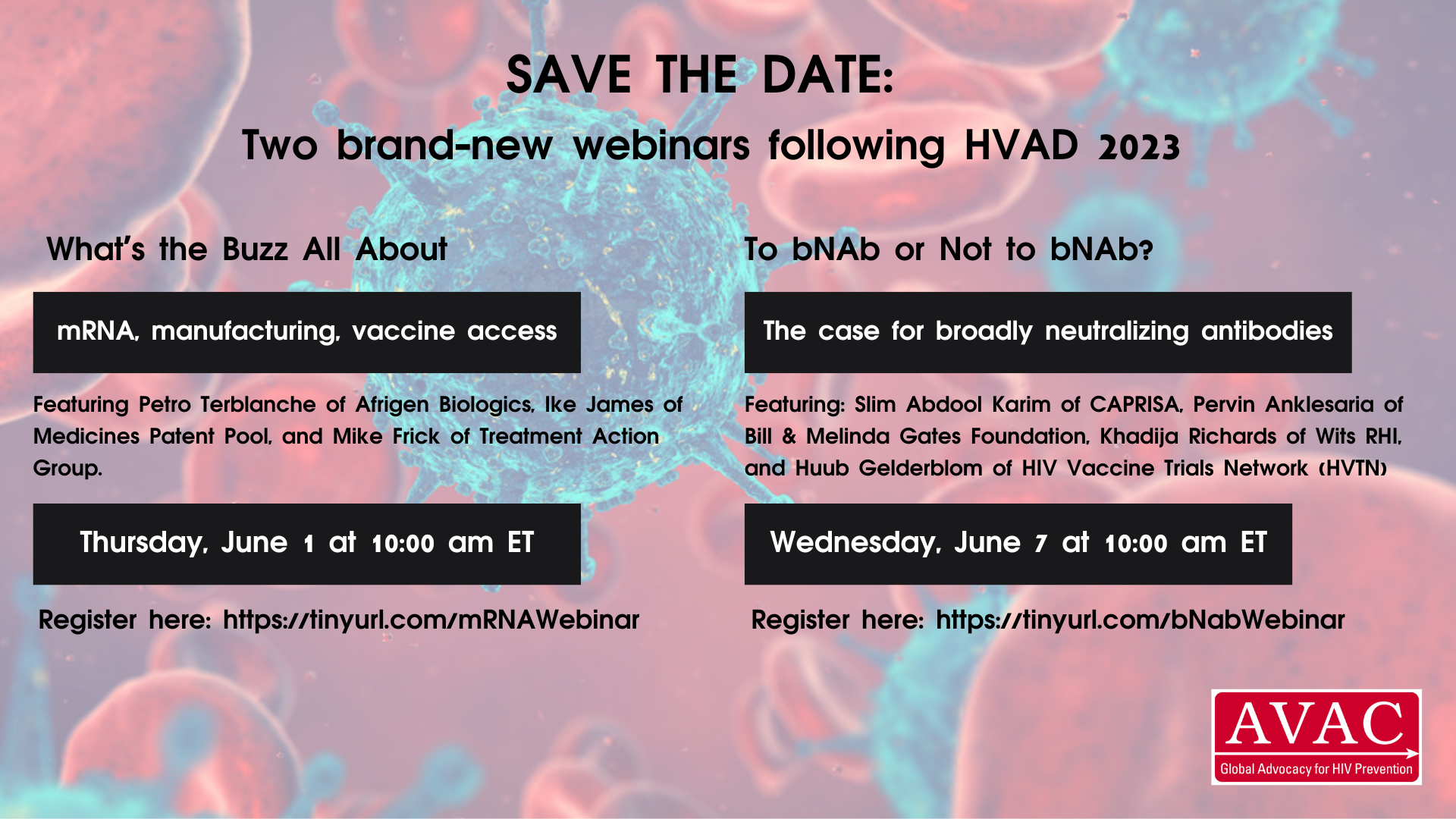
Webinars: AVAC brought together partners, advocates and other experts for two vaccine webinars. The first, on local manufacturing production, takes on this essential part of the solution for ensuring sustainable and equitable supplies of vaccines in low- and middle-income countries. The second explores the development of broadly neutralizing antibodies (bNABs) and their potential in HIV prevention. Check out the recording and slides for both.
What’s All the Buzz About: mRNA, manufacturing, vaccines access – featuring Caryn Fenner of Afrigen Biologics, Ike James of Medicines Patent Pool, and Mike Frick of Treatment Action Group.
“LMICs (Low- or Middle-Income Country) are not only the consumers of IP (Intellectual Property), we are the generators of IP!” Caryn Fenner, Afrigen Biologics
“It’s important for us in the Global North to know that vaccine technology didn’t start in Global North and was often taken from others.” Mike Frick, Treatment Action Group.
“Engagement and commitment [is needed], not only when a pandemic exists, but long-term commitments are key to follow through on.” Ike James, Medicine Patents Pool
To bNAb or not to bNAb? The case for broadly neutralizing antibodies – featuring Slim Abdool Karim of CAPRISA, Pervin Anklesaria of Bill & Melinda Gates Foundation, Khadija Richards of Wits RHI, and Huub Gelderblom of HIV Vaccine Trials Network (HVTN).
“The scientific complexities of bNAbs require a more attuned focus on community engagement… And it will require a high degree of validating [people’s] lived experience.”, Khadija Richards, Wits RHI.
“The hope is that if bNAbs are effective in prevention, they create a pathway towards a vaccine.” Slim Abdool Karim, CAPRISA
“To close the gap between scientific innovation and globally accessible and affordable bNAb combination products…we certainly need innovation in manufacturing. It is a long-term process and it’s not going to happen overnight. But we do need to start now.” Pervin Anklesaria, Bill & Melinda Gates Foundation
“What I see is actually multiple modes of PrEP available, and then people can make choices depending on what works for them.” Huub Gelderblom of the HIV Vaccine Trials Network on the potential of bNAbs as PrEP