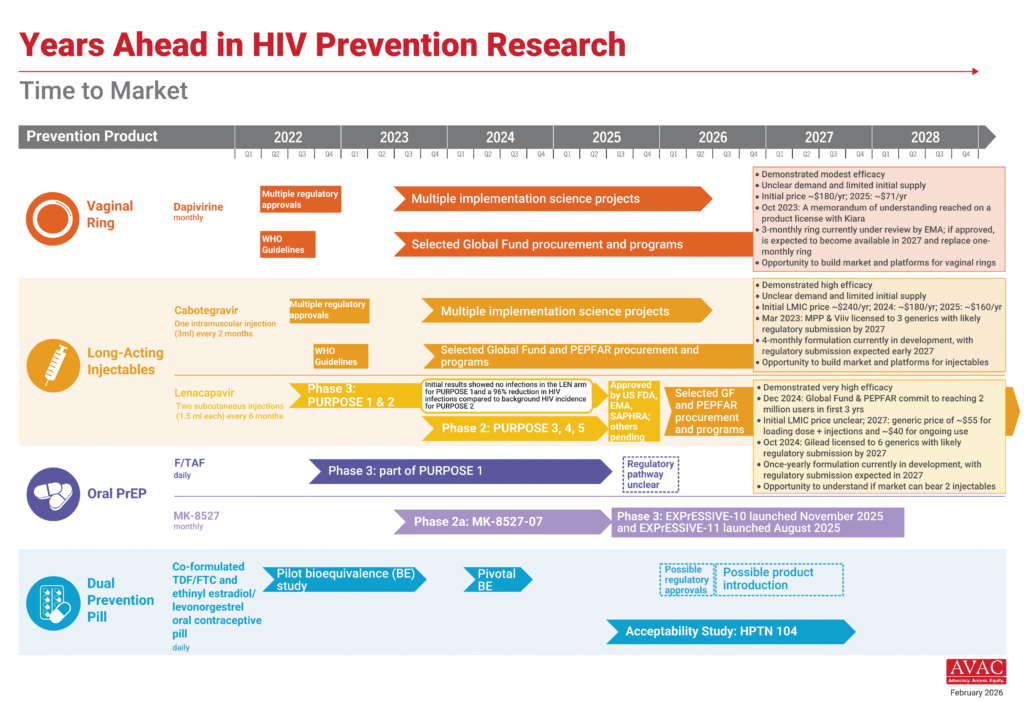
This week covers the significant turmoil at the US National Institutes of Health (NIH), including a close look at the NIH deputy director’s role in disruptions in leadership and funding cuts, and a lawsuit filed by the former Director of the National Institute of Allergy and Infectious Diseases (NIAID). It also covers urgent civil society pushback against efforts to sunset UNAIDS, the rapid expansion of US “America First” bilateral health agreements across Africa, and what’s next for STI research, prevention and diagnostics.
NIH Leadership Turmoil
A new investigation by The Atlantic’s Katherine Wu examines the central role of the Deputy Director of the National Institutes of Health (NIH), Matthew Memoli, in recent leadership changes and funding cuts across the NIH, including the firing of former NIAID Director, Jeanne Marrazzo, and the reassignment of Carl Dieffenbach, longtime director of NIAID’s Division of AIDS, to another NIH center. The Atlantic’s reporting shows how these decisions have significantly weakened NIAID, with particularly acute impacts on HIV prevention and vaccine research, where programs, expertise, and long-term scientific capacity are being eroded amid broader restructuring and budget shifts.
In addition, this week, Dr. Marrazzo, the newly appointed chief executive officer of the Infectious Diseases Society of America (IDSA), filed a lawsuit against the US federal government. The lawsuit alleges that Marrazzo was illegally fired from her position and seeks to be reinstated as head of NIAID and to receive formal declarations that her rights were violated. The suit argues that her firing was retaliation for a whistleblower complaint she filed on September 3, in which she raised concerns about anti-vaccine positions held by newly appointed NIH officials; demands to halt clinical trials; and requests to cut international research collaborations. Twenty-two days after filing the complaint, Marrazzo was fired by Health and Human Services Secretary, Robert F. Kennedy Jr.
IMPLICATIONS: The developments at the NIH show instability at its leadership level and severe consequences for infectious disease research, particularly vaccine research. This comes at a time when sustained R&D investment is most critical. Without robust research support, future advances in next-generation options could stall. See the new People’s Research Agenda, which calls for a balanced HIV prevention portfolio that is optimized for impact. However, Marrazzo’s new role at the IDSA, which represents clinicians, scientists and public health experts who are driving policy and advocacy to address critical issues in combating infectious disease, will be pivotal in ensuring rigorous science, inclusion and equity in research, community engagement, and evidence-based communications remain central to tenors in global health discourse and debate.
READ:
- AVAC Board Member Jeanne Marrazzo named CEO of IDSA—AVAC
- The Most Feared Person at the NIH Is a Vaccine Researcher Plucked From Obscurity—The Atlantic
- Fired NIH institute head sues Trump administration—Science
- IDSA selects next CEO—Infectious Diseases Society of America
Future of UNAIDS
Member states and civil society convened at the 57th meeting of the UNAIDS Programme Coordinating Board (PCB) in Brazil this week to discuss urgent decisions about the future of the global HIV response amid deep funding cuts and a shifting global health landscape. They reviewed and approved the Global AIDS Strategy 2026–2031 and assessed the impact on communities from disruptions to services for HIV prevention and treatment. Civil society representatives on the PCB shared comments, and African women leaders and other civil society groups issued statements (and sign-ons) denouncing efforts to sunset UNAIDS by the end of 2026: “Any move to sunset UNAIDS before ending AIDS as a public health threat is premature and unacceptable. No sunsetting until we finish the job. AIDS is not over.”
The PCB also held a special thematic session on long-acting ARVs for treatment and prevention. See this clip from Yvette Raphael where she included AVAC’s latest blog in her remarks about the current context: “We cannot let cruel international policy allow historic gains to collapse, just as a few highly effective prevention options arrive. That is why rolling out LEN to all countries that need it with speed, scale and equity must be our uncompromised, uncompromising priority. If we do this, we can change the trajectory of the epidemic, but only if we act at the pace that data and science demand.”
IMPLICATIONS: As the world moves toward the June 2026 High-Level Meeting on HIV, and the rollout of the new Global AIDS Strategy, the strong pushback by civil society at the PCB underscores that any reform must preserve UNAIDS’ core mandate and ensure that the global HIV response remains centered on those most affected — especially women, girls, and key populations — rather than being quietly dismantled at a moment of crisis.
READ:
- African Women Leaders Condemn Any Move to Sunset UNAIDS: AIDS is Not Over—African Women Prevention Community Accountability Board
- Deputy Secretary-General, Addressing UNAIDS Programme Coordination Board, Highlights Transition Strategy, Legacy as United Nations Success Story—Press UN
- The Future of HIV Prevention Depends on Speed, Scale and Equity—AVAC
- UNAIDS Must Be Retained as a Distinct, Independently Mandated Joint Programme within UN Reform—KP-TNC Statement
- The HIV Response Orphaned: At the Cost of Women Living with HIV—ICWEA
Eswatini and Mozambique Join Growing List of African Countries to Sign “America First” Bilateral Health MoUs
Mozambique and Eswatini are the latest countries to sign the bilateral health Memorandums of Understanding (MoUs) with the United States under the America First Global Health Strategy, adding to earlier agreements with Kenya, Liberia, Rwanda and Uganda. These agreements provide 5 to 10 years of funding and health support in exchange for co-financing, health data, pathogen-specimens, and national health system data, marking a major shift in how global health cooperation is structured under US leadership.
IMPLICATIONS: The pace of these agreements shows how quickly the Administration is moving to reshape US global health with little time for discussion and debate on transparency, consultation, and how civil society fits within this evolving framework. As the landscape shifts, civil society and non-governmental organizations must quickly reassess their roles in the hope of preserving aid delivery, accountability, and equity.
READ:
- NGOs must prove relevance to survive in ‘America First’ health strategy—Devex
- Far from over—Forsaken Substack
- US-Africa Health Agreements Targets Don’t Add Up—To End a Plagues … Again Substack
The Future of HIV Prevention Depends on Speed, Scale and Equity
“Every funding cut can represent at minimum, a delay. Every delay in rollout is a missed chance to prevent infections. Every un- or underfunded clinic is a barrier to access. Every policy is a choice to be inclusive or leave someone behind…”
What We’re Reading
- Reimagining HIV prevention programmes: the time is right, but what needs to be done?—The Lancet HIV
- Is science diplomacy still possible?—The Lancet
2025 Broke the System. 2026 Decides the Future of Global Health.—Lights.Camera.Equity Substack - When politics Trumps science: Why the US isn’t giving South Africa LEN—Financial Mail
- Will New US Aid Strategy Improve Countries’ Self-Reliance?—Medscape
- World leaders adopt a historic global declaration on noncommunicable diseases and mental health—WHO
- Forget quiet quitting, the State Department appears to be quiet hiring—Devex
- Trump Officials Celebrated With Cake After Slashing Aid. Then People Died of Cholera.—ProPublica
- Exclusive: Senate Democrats introduce bill to protect UN Population Fund—Devex
- California Hires Former C.D.C. Officials Who Criticized Trump Administration—New York Times
- Update on Lives Lost from USAID Cuts—CGD
- U.S. Global Health Country-Level Funding Tracker—KFF

STIs Spotlight
AVAC’s STI program looks back at 2025—and ahead to 2026—tracking rising STI rates, major HPV vaccination gains, slow but promising diagnostics, the growing role of self-care, and what’s next for STI research, prevention and diagnostics.