This toolkit for researchers shares key messages, practical advocacy guides, and resources to help move our collective efforts forward.
Research Matters Advocacy Toolkit
Despite USG Global Health Collapse, Here Are Several Data Trackers To Support Your Advocacy
With the collapse in support from the US Presidential administration in global health, data sources tracking the HIV response have been lost, from HIV incidence and prevalence to PrEP uptake and disparities among key populations and regions of the world. Below, AVAC has identified dashboards, data trackers and other resources to inform your advocacy.
Tracking PrEP Access
- Impact of the Stop-Work Order on PrEP
On PrEPWatch, this webpage outlines the consequences of PEPFAR stop-work orders on HIV prevention, detailing disruptions to PrEP services, stalled product rollouts (including CAB), halted research, and workforce reductions. It includes downloadable slides to support advocacy and analysis.

- Tracking Lenacapavir Rollout
This new online tracker monitors all the key steps, timelines and responsible stakeholders needed to ensure equitable access to injectable lenacapavir (LEN) for PrEP. Find it on PrEPWatch.

Tracking the Impact of US Government Funding Cuts
- Tracking the Freeze: Real-Time Impact on Key Populations
The Global Black Gay Men Connect (GBGMC) network, with more than 150 partners across regions, is tracking the immediate and devastating effects of USG freeze on foreign assistance. This page compiles verified data from the field and updates it weekly.
- PEPFAR Funding Freeze
This webpage shares resources developed by a new global civil society coalition, Community Health and HIV Advocates Navigating Global Emergencies (CHANGE) on various impacts of the freeze.
- Impact of Partial PEPFAR Funding Freeze and Discontinuation: Estimated Deaths
Impact Counter’s tracker reflects the estimated number of deaths that have occurred between January 24, 2025, and today, as a result of the partial funding discontinuation. (See also Medicaid Impact Counter & Tuberculosis Impact Tracker.)
- Impact of US Funding Cuts on the Global AIDS Response
UNAIDS’ weekly situation report provides an overview and up-close country snapshots of the impact to service delivery and human resources.
- The Best Investment You Didn’t Know You Made
How NIH Funding Fuels Innovation and Economic Growth, and other publications from amfAR. - HIV Market Impact Memo
This brief from CHAI shares significant risks facing global HIV markets and the broader implications for HIV service delivery in LMICs following global health funding disruptions and ongoing uncertainty. - The USAID List of Terminated Global Health Awards – What Does it Tell Us?
This resource from KFF shares a list of USAID awards and their status—either active or terminated.
Tracking Court Action
- Litigation Tracker: Legal Challenges to Trump Administration Actions
JustSecurity.org tracks legal challenges to the administration’s actions. - AVAC V. United States Department of State
AVAC’s dedicated page shares updates on the case against the US government seeking emergency relief from an Executive Order that inhumanely froze all funding for foreign assistance. Our latest episode of PxPulse Live, Lawsuit Wins and What’s at Stake: AVAC v US Department of State offers context and updates.
These trackers and additional resources are included in our weekly Global Health Watch newsletter along with the latest policy developments and their implications to keep advocates informed, prepared and connected.
Global Health Watch: Tariffs, NIH Cuts, Black-led HIV Research Agenda & PEPFAR’s Legacy
This week brought major developments for global health: new tariffs on pharmaceuticals are pending, a court blocks the cap on NIH indirect costs, and worries a leadership vacuum at the CDC is a cause for yet more concern. Amid the chaos, advocates rallied—defending PEPFAR’s legacy in Congress and launching a national Black-centered, Black-led HIV research agenda.
Read on for highlights and implications and be sure to check out the What We’re Reading section, which is full of great pieces this week.
Tariffs and HIV
As the administration created even more chaos with the on-again, off-again sweeping tariffs and threats of major trade wars, a new report highlights concern and potential effects on health systems—including HIV prevention and care. Finished pharmaceuticals are temporarily exempt, but essential components like diagnostic tests, syringes, excipients and other medical supplies may not be protected, raising alarms about cost increases and supply chain delays. And on Tuesday, the President announced at a dinner that new tariffs targeting pharmaceuticals are now officially “coming soon.”
IMPLICATIONS: If global pharmaceutical manufacturers move their operations to avoid tariffs, FDA inspections—with many fewer resources in the wake of last week’s mass layoffs—could delay approval of new products. Clinics, hospitals and other health systems may face increased costs, limited availability of products and a more fragile supply chain.
READ:
- How Will Trump’s Tariffs Impact Medicine and Healthcare?—Medpage Today
- Trump Defends Tariffs, Announces Pharmaceutical Levies Coming Soon—The Wall Street Journal
NIH Overhead Cuts Blocked by Court
A federal judge issued a permanent injunction blocking an administration policy that would have capped indirect cost payments at 15% for both new and existing NIH grants. The policy threatened to cut billions in support for universities, academic centers, and research institutions—jeopardizing infrastructure, staff, and ongoing studies. While the administration may appeal the ruling, it marks an important step in what could be a long legal battle over the future of federal research funding. At the same time, massive uncertainty remains at NIH, given the numerous staff and grant terminations.
READ:
- Federal judge issues permanent injunction on Trump cuts to research overhead payments—STAT
- A closer look at the nationwide impact of NIH cuts—Axios
- How Trump 2.0 is slashing NIH-backed research — in charts – Nature
US CDC Uncertainty
Internal memos reported by Inside Medicine indicate that the US CDC currently has no legally-required Acting Director, which leaves the agency in a leadership vacuum at a critical time. Dr. Susan Monarez, who previously served in an acting capacity, became ineligible for that role after being nominated for the permanent position on March 24. In the meantime, scientists and advocates are calling on federal and state health leaders to protect the nation’s only STD reference laboratory and reinstate over 30 scientists affected by the recent reduction in force (RIF) amid a growing public health crisis of rising STDs and drug-resistant infections. Colleen Kelley, chair of the HIV Medicine Association (HIVMA) testified before Congress Wednesday advocating for the CDC’s prevention division, continued funding in HIV care, prevention and research.
IMPLICATIONS: Without a legally authorized director, decisions normally reserved for CDC leadership—including the acceptance of upcoming vaccine recommendations by the Advisory Committee on Immunization Practices (ACIP)—must now be made by HHS Secretary Robert F. Kennedy Jr., a known vaccine skeptic. This raises urgent concerns about legal compliance, scientific integrity, and public trust, particularly as thousands of CDC staff have been laid off and critical public health decisions loom.
READ:
- Scoop: CDC has no Acting Director, sources confirm—Inside Medicine
- A Federal Lab That Tracked Rising S.T.I.s Has Been Shuttered—New York Times
Making the Case for PEPFAR
On Tuesday, EGPAF’s Catherine Connor and Ambassador Mark Dybul testified at the US House Appropriations Subcommittee hearing on PEPFAR, issuing powerful affirmations of the program’s life-saving impact—and the bipartisan commitment to its future. Lawmakers from both sides showed strong support for PEPFAR. They also shared an interest in innovation—including the promise of long-acting PrEP—to strengthen the program’s next phase. Their testimony came at the same time that Michel Sidibe and colleagues published new data in a Lancet Correspondence underscoring PEPFAR’s legacy—in saving an estimated 26 million lives, and also in catalyzing a 212% increase in domestic health investment across PEPFAR-supported African countries, since 2004. In the same issue, Lucie Cluver published updated modeling of the impact of potential PEPFAR cuts.
READ/WATCH:
- Hearing – Assessing the President’s Emergency Plan for AIDS Relief (PEPFAR)—House Appropriations Committee
- Accelerating domestic investments to end AIDS in Africa—The Lancet
- Protecting Africa’s children from extreme risk: a runway of sustainability for PEPFAR programmes—The Lancet
- Impact of PEPFAR Stop-Work Order on PrEP—AVAC
A Black-led Agenda for HIV Research
PrEP in Black America (PIBA) and more than 80 Black researchers, scientists, and community leaders, launched the first-ever national Black HIV Prevention Research Agenda this week, a call to action and a blueprint to end HIV in Black communities. The agenda centers Black voices, leadership, and lived experience to influence how HIV prevention research is conducted, funded, and implemented. AVAC’s John Meade described the launch as a moment of “reckoning and resistance,” pointing to the urgent need to protect public health infrastructure, advance equity, and resist political threats to HIV research and LGBTQ+ rights. This domestic research agenda importantly complements the People’s Research Agenda that AVAC and global partners released last October. The two documents provide a truly global, community-led perspective on the future of HIV prevention research.
READ:
- Advancing the Movement: HIV Prevention Research For Black Communities—PIBA
- Advancing the movement: 100 Black researchers, scientists unveil agenda to support HIV Prevention Research—The Atlanta Voice
The Future of Injectable Lenacapavir for PrEP
Clinical Infectious Diseases covered two viewpoints offering different perspectives on the future of injectable lenacapavir for PrEP—and the future of HIV prevention more broadly.
- The Need for Lenacapavir Compulsory Licences in Ending the HIV Epidemic
- Lenacapavir for Human Immunodeficiency Virus (HIV) Prevention: A Commitment to Equitable Access and Partnership by Gilead Sciences
What We’re Reading
- Is This the Beginning of a New H.I.V. Crisis?—The New York Times
- Undermining HIV Prevention Now Will Cost Billions Later—Contagion Live
- Who Will Care for Infants With H.I.V. Overseas—The New York Times
- A refusal to abandon HIV science—AIDS
- The Effects of Reductions in United States Foreign Assistance on Global Health—SSRN (pre-print)
Update on AVAC vs. Department of State
Two months ago, AVAC sued the US government over an Executive Order that froze all foreign assistance.
Since then, the court has ordered the government to restart certain payments and uphold its legal obligations. But delays, resistance, and appeals continue—putting global health, HIV prevention, and US credibility on the line.
Read our update on the case and watch our latest episode of PxPulse Live where AVAC’s Executive Director Mitchell Warren and Public Citizen litigator Lauren Bateman unpack the latest legal developments.

Resources
- The Status of PEPFAR’s USAID Programming—amfAR
- Tracking the Freeze:Real-Time Impact on Key Populations--GBGMC
- The impact of suspensions and reductions in health official development assistance on health systems—WHO
- The Best Investment You Didn’t Know You Made: How NIH Funding Fuels Innovation and Economic Growth— amfAR
- Quick Take: HIV Research Matters for America—O’Neil Institute and amfAR
- What Do Federal Staffing Cuts and HHS Restructuring Mean for the Nation’s HIV Response?—KFF
- Domestic Funding Contributions to Health: Comparing Changes in Domestic Financing in PEPFAR and Non-PEPFAR Supported Countries—amfAR
- The USAID List of Terminated Global Health Awards – What Does it Tell Us—KFF
Impact of PEPFAR’s Stop Work Order on PrEP
The impact of the stop-work order on PrEP is expected to be severe. In a set of slides and on our website, PrEPWatch, we have posted the results of an analysis drawing on key informant interviews with representatives of Ministries of Health and PrEP implementers between 27 January 2025, when stop-work orders were issued by the US government, and the end of February 2025, when the vast majority of USAID-funded projects received official termination notices. Find the latest here.
Trials & Projects Halted by USAID Funding Suspension
The stop-work orders have disrupted USAID-supported HIV prevention research, halting critical investigations in vaccine and next-generation PrEP strategies.
The abrupt suspension of these trials also raises serious ethical concerns. Stopping trials mid-course undermines trust in research, jeopardizes community engagement, and abandons participants who volunteer their bodies for scientific discovery. It will take years to build back this critical infrastructure—for HIV research and beyond—as well as the community partnership and trust needed to ensure smooth and ethical research.
HIV Incidence, Age 15-49
Looking backward and then into the future, this chart shows actual HIV rates alongside projected rates with and without current prevention strategies (PrEP, VMMC, and free condoms).
Global PrEP Uptake and PEPFAR’s Role, 2016-2024
AVAC’s Global PrEP Tracker has documented cumulative PrEP initiations on a quarterly basis for nearly a decade. This graph presents the final data collected while PEPFAR was fully operational—PEPFAR support was responsible for 79% of PrEP uptake globally in the last year and reached 83% by the end of September of 2024. Data on the fourth quarter of 2024 is inaccessible since PEPFAR was taken offline in late January.
PxWire Volume 15, Issue No. 1
In this special edition of Px Wire, AVAC is going beyond a quarterly update of biomedical HIV prevention. In this issue, we look at how the new US Administration’s attack on global health can be expected to devastate HIV prevention, including the capacity to deliver existing PrEP options, the scale up of new PrEP products, and the paralyzing impact on research and development. A PDF version of this report is also available.
From Research to Rollout: The impact of USG global health pullout


The United States’ presidential regime has launched a sustained, multi-pronged attack against foreign assistance, scientific inquiry, due process and good governance. It threatens economies, human rights, international partnerships, global health at large, and the rule of law. For HIV prevention, a single sentence, issued in a February 6 advisory from the US Department of State, has derailed the entire field, potentially setting back the HIV response by years, if not decades. Read on for resources to support your advocacy and fortify our solidarity at this critical time.
Progress in PrEP Uptake: Threatened
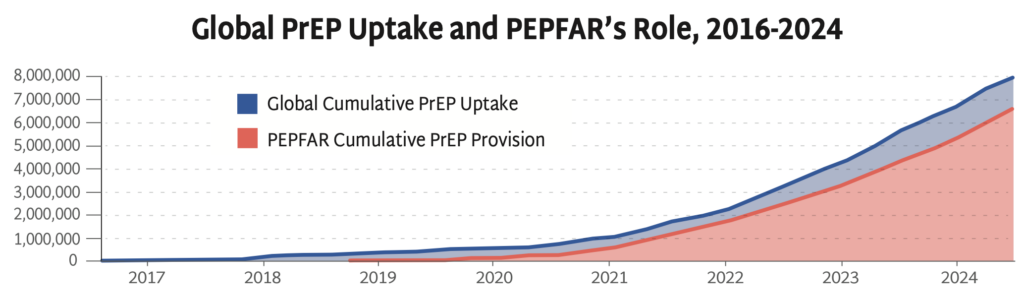
PEPFAR has been pivotal to accelerating PrEP uptake, significantly expanding HIV prevention coverage. The freeze on foreign aid prohibits funding to PEPFAR’s PrEP programs and poses a serious threat to global efforts to control the epidemic.
AVAC’s Global PrEP Tracker has documented cumulative PrEP initiations on a quarterly basis for nearly a decade. This graph presents the final data collected while PEPFAR was fully operational—PEPFAR support was responsible for 79% of PrEP uptake globally in the last year and reached 83% by the end of September of 2024. Data on the fourth quarter of 2024 is inaccessible since PEPFAR was taken offline in late January.
At the time of the foreign aid freeze, PrEP uptake had reached 8 million initiations since 2016, an achievement that’s taken almost 10 years to reach—too slow and too small to reach UNAIDS targets, but a robust foundation to finally accelerate PrEP uptake with next-generation PrEP. Current US policies, instead of expanding PrEP coverage, are leading to the closure of programs, and will reverse global progress against HIV.
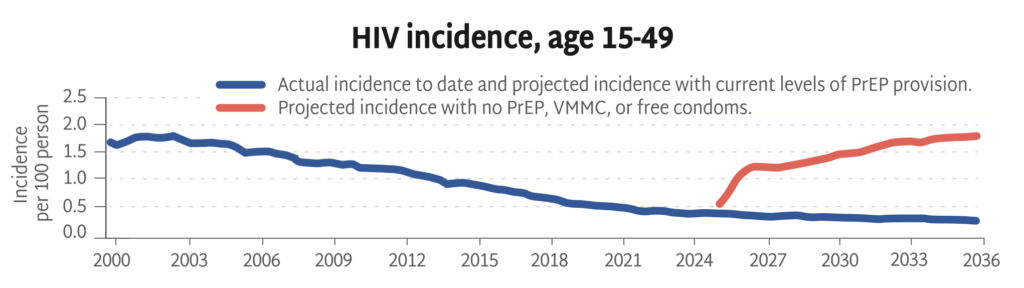
Without primary prevention, the HIV epidemic is poised to rage on, with incidence among adults on track to triple over the next ten years. This HIV Synthesis model, developed by the HIV Modelling Consortium, estimates the impact of stopping all HIV prevention services across Africa from now through 2036—including PrEP, voluntary medical male circumcision (VMMC), and free condom distribution.

For the last 8 years, AVAC has proudly worked with PEPFAR to document PrEP uptake and its impact around the world. That stopped in January with a stop work order from the US government. But protecting access to PrEP is vital. Are you leading a PrEP program? Whether supported by PEPFAR or not, we invite you to work with us to ensure global data on PrEP is not lost. Find us at [email protected].
PrEParing for New Products: Is rollout still possible?

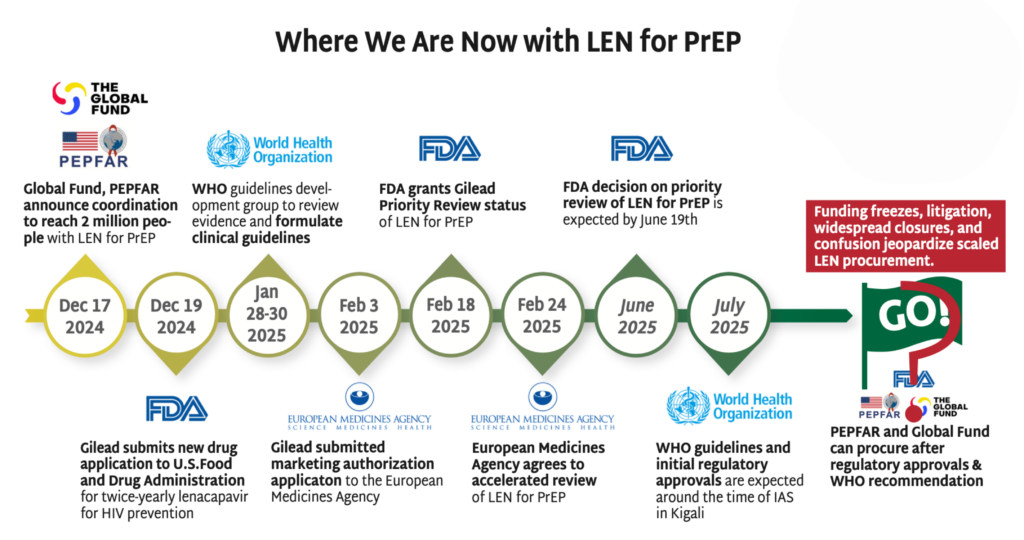
Read more in The Gears of Lenacapavir for PrEP Rollout.
The chaos in foreign assistance programs (including discontinuation of major PrEP programs), cuts in staffing and new demands on donor commitments will make decisions on the procurement of LEN for PrEP more complex and uncertain. In December 2024, the Global Fund and PEPFAR announced a plan to reach 2 million people with LEN for PrEP over three years. Exactly how funding to support this unprecedented introduction program will move forward, in the absence of significant US investment, is far from certain. The other stakeholders, including Global Fund, Gilead, CIFF and the Gates Foundation expressed commitments to the deal, but major questions remain. In the meantime:
- Gilead’s production of LEN for PrEP is continuing, as is the technology transfer to generic license holders.
- The FDA granted Gilead priority review status for LEN for PrEP, with a decision due by June 19, 2025.
- The EMA has agreed to an accelerated review of LEN for PrEP for both European access and as part of the EU-Medicines for all (EU-M4all) program, reducing the review from seven months to five months.
- The WHO’s Guideline Development Group (GDG) met in January, and WHO is expected to issue guidelines by July 2025.
The Latest R&D in the Prevention Pipeline: Supported or undermined?

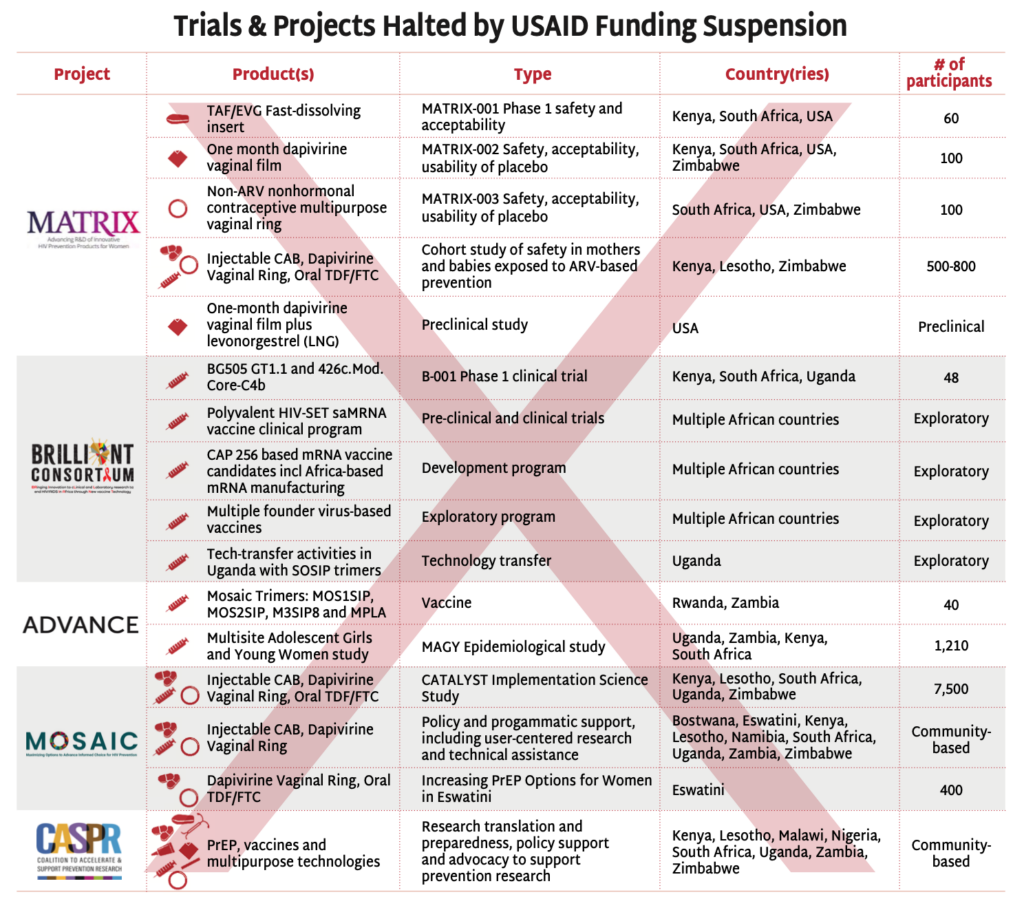
The stop-work orders have disrupted USAID-supported HIV prevention research, halting critical investigations in vaccine and next-generation PrEP strategies.
- The BRILLIANT and ADVANCE projects’ clinical, preclinical, and experimental trials testing HIV vaccine candidates have been suspended.
- The MATRIX projects’ driving innovation with next-generation PrEP and MPT products, fast-dissolving inserts and vaginal films and rings, have been forced to stop their clinical trials.
- The MOSAIC projects’ have suspended all implementation science activities, including the CATALYST study, investigating choice among oral PrEP, injectable cabotegravir and the dapivirine vaginal ring. Other implementation studies are continuing, but access to the commodities, much of which was procured by PEPFAR is questionable. See AVAC’s Integrated Study Dashboard for details.
- The Coalition to Accelerate and Support Prevention Research (CASPR) has also been paused. Led by AVAC in partnership with a number of leading African civil society organization, CASPR focuses on building an enabling environment for HIV prevention R&D. (Note: In early February, AVAC lead a lawsuit against the State Department seeking emergency relief from the freeze on foreign assistance, including funding for CASPR. The case, AVAC v. United States Department of State, is pending.)
These disruptions delay the development of urgently needed HIV interventions and threaten the sustainability of research infrastructure all over the world, with particularly egregious impacts on the research capacity of regions most impacted by the epidemic.
The abrupt suspension of these trials also raises serious ethical concerns. Stopping trials mid-course undermines trust in research, jeopardizes community engagement, and abandons participants who volunteer their bodies for scientific discovery. It will take years to build back this critical infrastructure—for HIV research and beyond—as well as the community partnership and trust needed to ensure smooth and ethical research.
Prevention Playlist
AVAC develops a wide range of resources to inform decision making and action. Check out the latest:
Join
- Subscribe to Global Health Watch: AVAC’s weekly newsletter dedicated to breaking down critical developments in US policies and their impact on global health. avac.org/signup
- Seeking Visuals and Videos: Leading groups in Washington, DC, are urgently trying to collect videos and photos documenting the impact of the US government’s foreign aid freeze, such as clinic closures despite the waiver. Non-professional phone videos and photos are welcome. Send to [email protected] for more details
- CHANGE: In response to the unfolding crisis, more than 1,300 people from civil society organizations around the world have launched CHANGE—Community Health & HIV Advocate Navigating Global Emergencies—a coalition formed to support urgent action. [email protected]
Use
- Graphic of studies of injectable cabotegravir and the dapivirine vaginal ring in eastern and southern Africa, AVAC
- Most Lifesaving Services Remain Paused: A Rapid Assessment of the PEPFAR Stop Work Order, amfAR, CHANGE, Data ETC
- What Effect are HIV Programmes Having in Africa, The HIV Modelling Consortium
- PEPFAR & Global Fund Support for HIV Programs, amfAR & Data ETC
Watch/Listen
- [LISTEN] Would PEPFAR Survive Trump—and what would it look like?
- The Impact and Implications of Recent US Government Federal Funding Reductions on Health Programmes, The Steve Biko Centre for Bioethics at The University of the Witwatersrand event recording
Read
- AVAC v United States Department of State. On February 10, 2025, AVAC and another nonprofit organization sued the new US Administration, seeking emergency relief from an Executive Order that inhumanely froze all funding for foreign assistance, AVAC
We’re Going Back to Court
Three weeks ago, AVAC, as well as the Global Health Council and partners, sued the U.S. State Department and government officials including the President, to end the freeze on foreign assistance funding that is harming global health and development programs, including lifesaving HIV prevention efforts.
On February 13, a US federal district court immediately granted a temporary restraining order and directed the government to lift the freeze and restore funding while our lawsuit progresses. Since then, the administration has repeatedly refused to restart funding, and the court has made clear more than once that the continued freeze is unlawful.
“The government comes to this Court with an emergency of its own making,” our lawyers wrote in a filing.
The lengths that the government is willing to go to flout a court order, all for the goal of ending life-saving humanitarian assistance, is staggering,” they said.
Now we have a court date: this Thursday, March 6 at 2pm Eastern Standard Time, we will be in Washington, DC, to make the case for human rights, health and dignity – and the government paying its bills.
This fight is far from over, and your support has been instrumental in reaching this point, and together, we can continue to champion the rights and health of communities worldwide.
How you can stay connected:
- Follow: For updates on our case and one filed by Global Health Council, visit AVAC vs. Department of State. For the latest on the policy environment in the US, subscribe to our weekly Global Health Watch.
- Donate: Your financial contributions are vital to fund our legal actions and advocacy efforts! Give here.
- Advocate: For the latest breaking news on US government developments, Join Community Health & HIV Advocates Navigating Global Emergencies (CHANGE) for briefings and advocacy strategizing. You can keep a close eye on implications at PEPFARWatch and the PEPFAR Impact Tracker.
- Share: Help us spread the word! Share Global Health Watch and other AVAC updates with your networks.
These are immensely challenging times for all of u, and it is easy to be paralyzed, overwhelmed and depressed. But we’ve all come too far for that to be the new normal. Lives, economies and democracies depend on our collective ability to stand up and fight back.
New Product Introduction Update
A graphic showing ongoing studies of injectable cabotegravir and the dapivirine vaginal ring in eastern and southern Africa.