In 2024, Gilead Sciences released findings from the PURPOSE 1 and PURPOSE 2 trials testing lenacapavir (LEN) as HIV prevention. The PURPOSE 1 trial found 100% efficacy in preventing HIV in 5,300 cisgender women in Uganda and South Africa, and the PURPOSE 2 trial showed a 96% reduction in HIV incidence among cisgender men, trans, and non-binary individuals across multiple countries. Both trials demonstrated LEN’s safety and effectiveness in reducing HIV transmission. In June 2025, the FDA approved injectable LEN for PrEP.
In September 2025, the Gates Foundation and Unitaid committed to accelerate access to generic versions of LEN on the heels of the Global Fund and PEPFAR re-committing to their December 2024 announcement of reaching two million people with LEN for PrEP within three years, with drug supplies coming from the originator company, Gilead Sciences.
The field is moving faster than the first decade of oral PrEP and the rollout of injectable cabotegravir, propelled by exemplary advocacy and improved coordination among stakeholders. But making lenacapavir, and new prevention options, available to all who need it requires even greater speed, scale, and equity. Ongoing community-led strategic action must continue and accelerate.
Find critical resources to inform your advocacy below:
Infographics
Download the static version of this graphic or hover over the graphic and click the three dots on the upper right to access full screen mode.

The gears framework for lenacapavir scale-up brings together a coalition of essential stakeholders, each contributing to the successful, sustainable integration of this HIV prevention tool into global health systems.
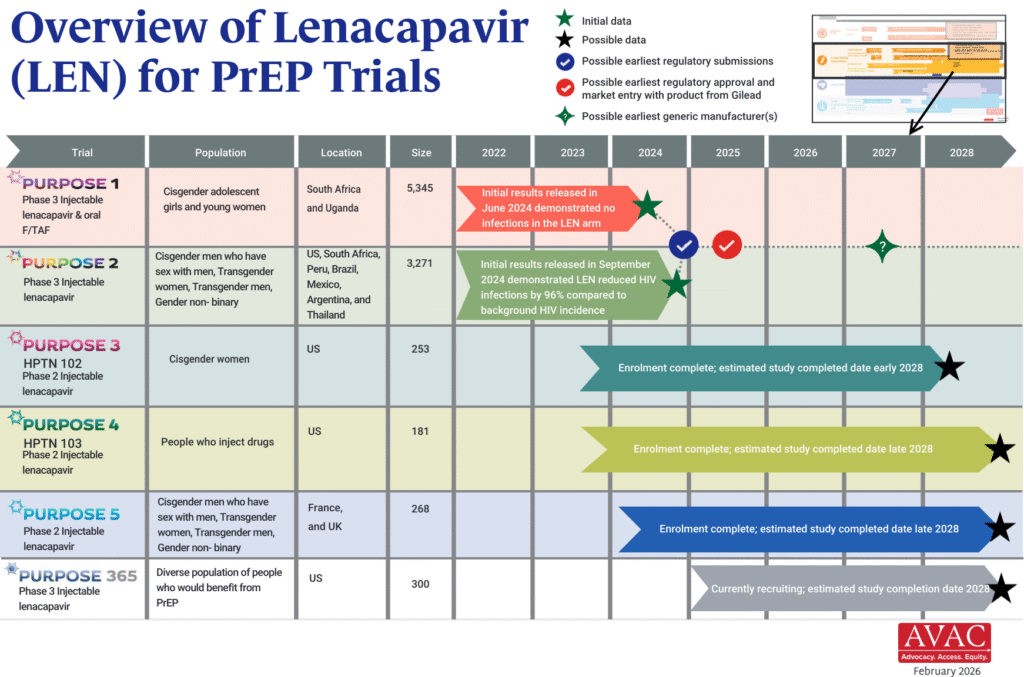
The PURPOSE trials evaluate the safety and efficacy of injectable lenacapavir (LEN), an investigational antiretroviral (ARV) drug being studied as a potential PrEP product. This graphic shows the latest status of all five trials including the groundbreaking results of PURPOSE 1 and PURPOSE 2.
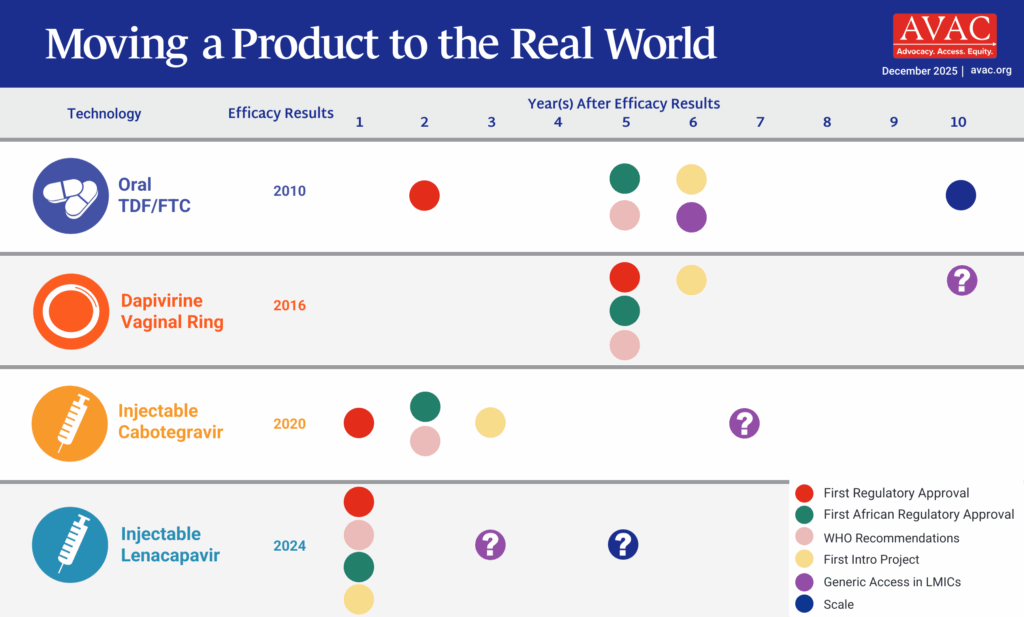
To reach the UNAIDS target of 10 million PrEP users by 2025, initiations of oral PrEP alone will not be enough—and this graphic shows that the field is beginning to apply past lessons to accelerate introduction of injectable cabotegravir.
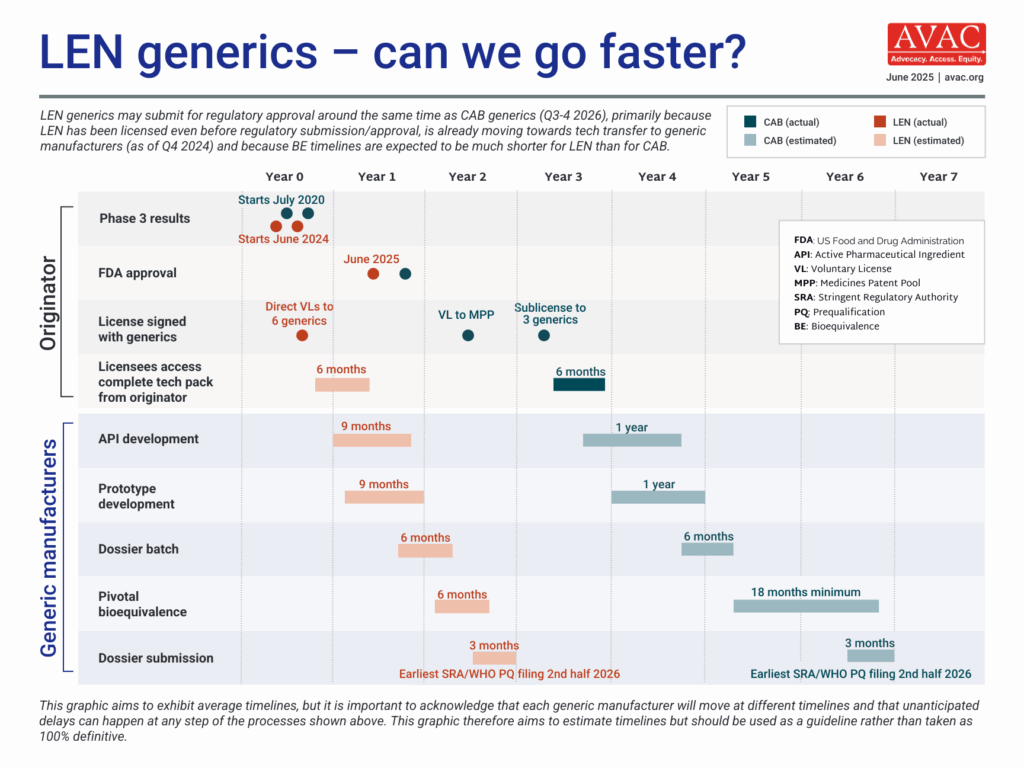
The timeline for generic LEN for PrEP to come to market is expected to be significantly shorter than for CAB for PrEP. Bioequivalence (BE) testing for LEN, which demonstrates a generic product works in the body in the same way as the originator product, is likely to be six months, vs. the 18 months for CAB for PrEP, because of differences in the drug formulation.
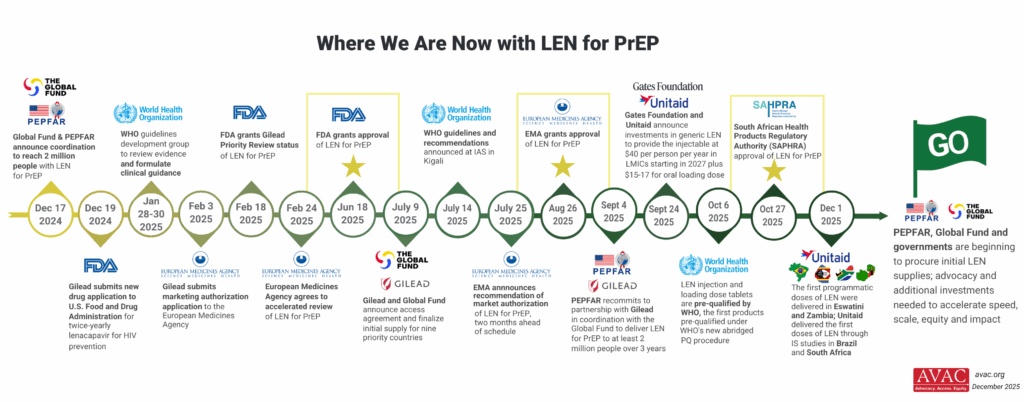
This timeline tracks the regulatory approval process for lenacapavir (LEN) for HIV prevention from late 2024 through 2025. Key milestones include FDA Priority Review status, FDA approval in June 2025, and expected subsequent approvals from European regulators and WHO.
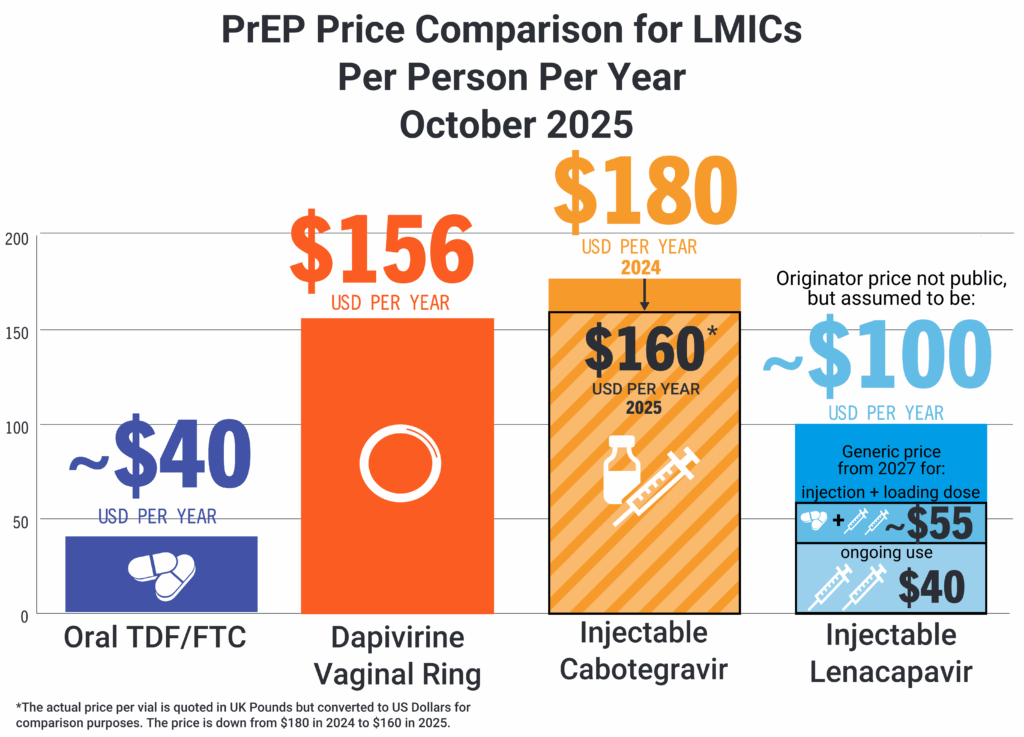
Comparing the annual price of oral TDF/FTC vs. the dapivirine vaginal ring and injectable cabotegravir.
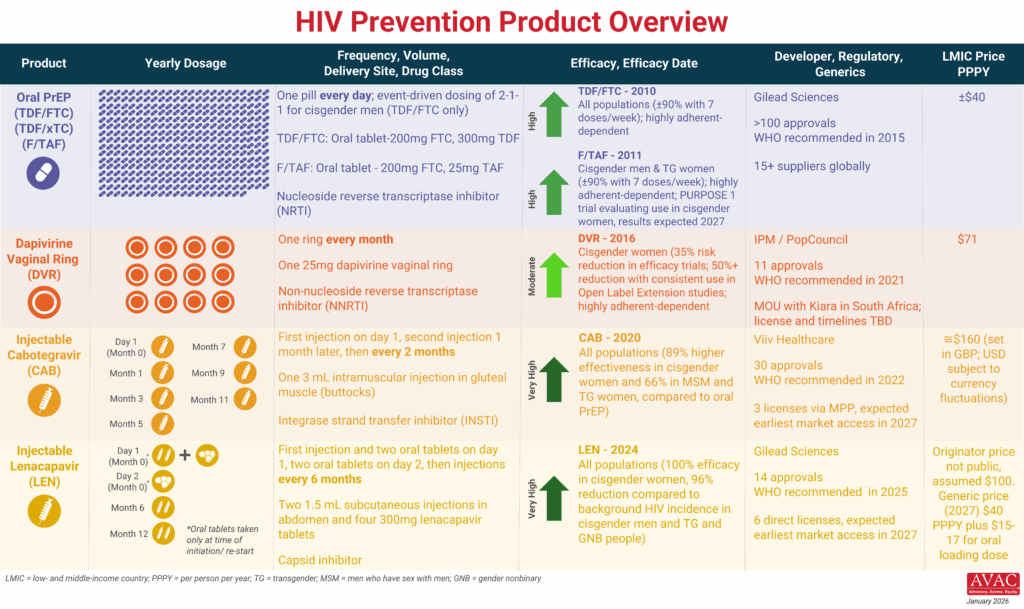
The graphic provides an overview of PrEP products currently available and in late-stage clinical trials.
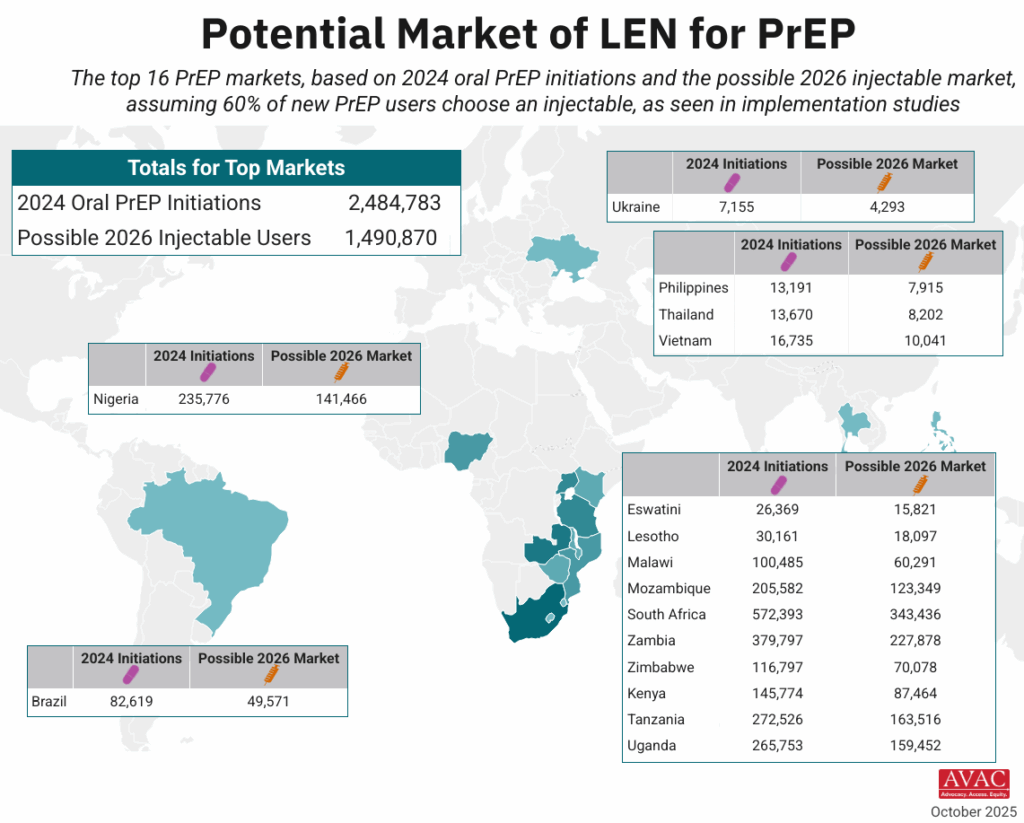
The top 16 PrEP markets, based on 2024 oral PrEP initiations and the possible 2026 injectable market, assuming 60% of new PrEP users choose an injectable, as seen in implementation studies.
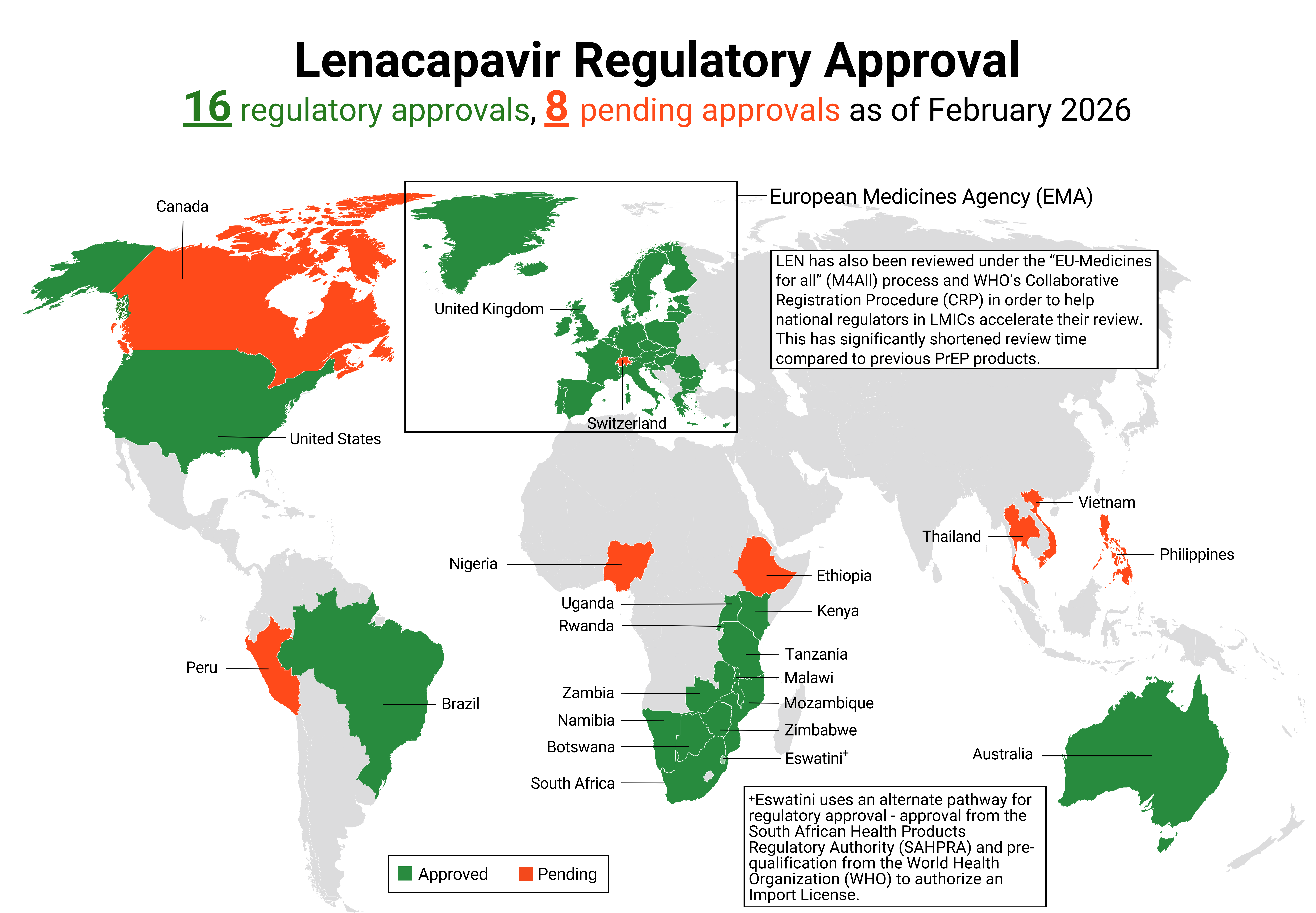
Regulatory approvals, pending decisions, and appeals as of February 2026. For product approvals, volumes, implementation, and price comparisons of long-acting PrEP, visit our dashboard on PrEPWatch.org.
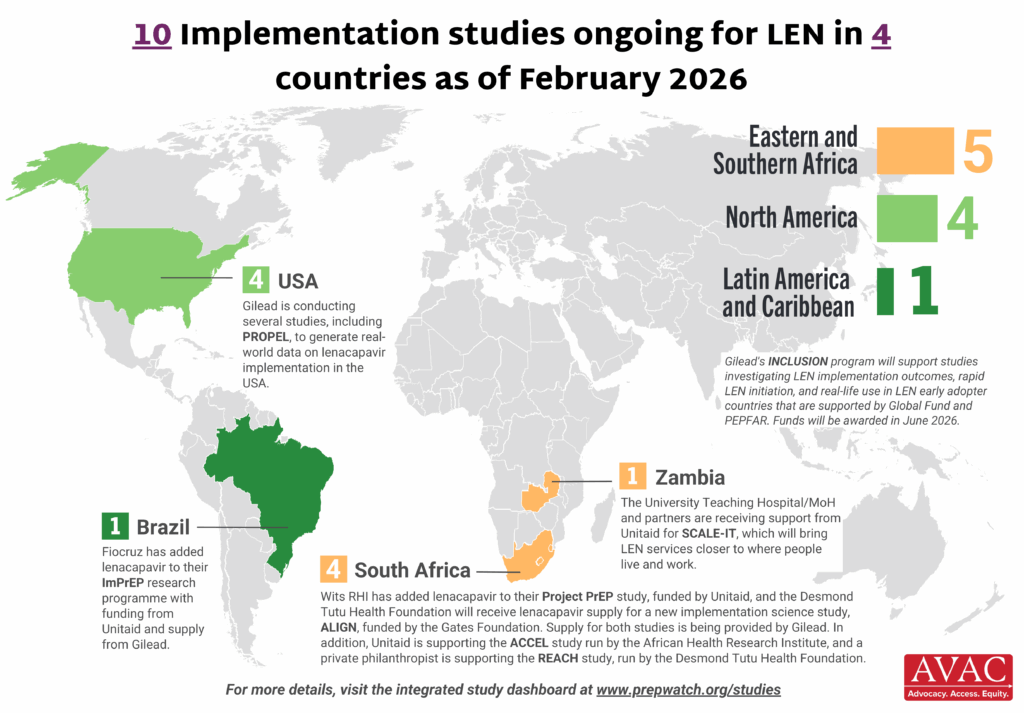
Ongoing and planned implementation studies for the lenacapavir as of October 2025. For product approvals, volumes, implementation, and price comparisons of long-acting PrEP, visit our dashboard on PrEPWatch.org.
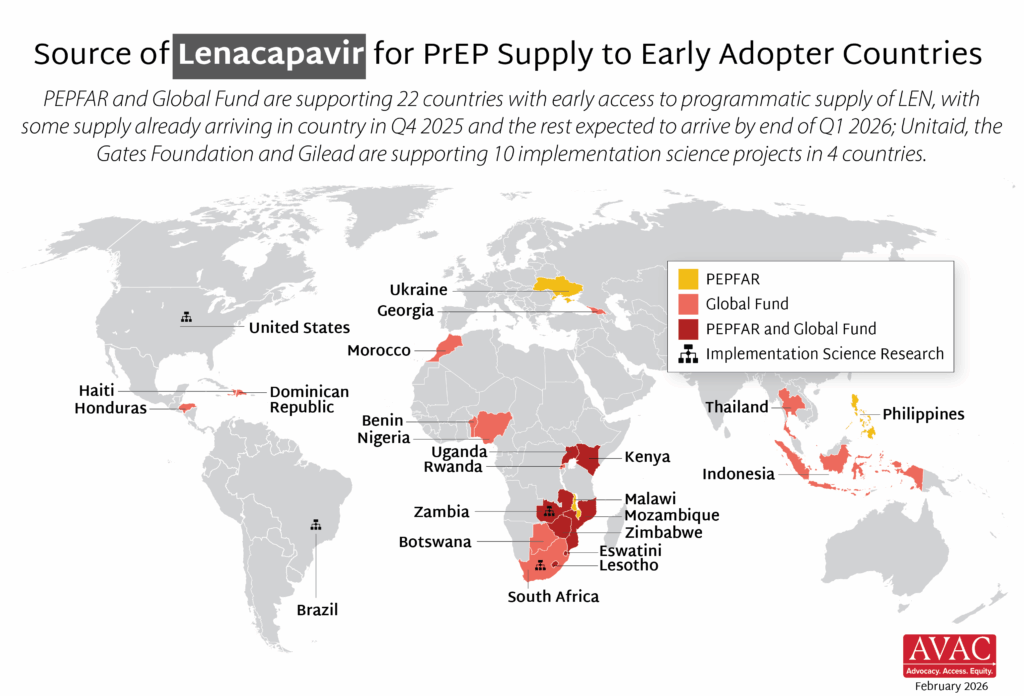
The Global Fund, with support from CIFF, and PEPFAR have jointly committed to reaching up to two million people with LEN for PrEP over three years. Supply of LEN is due to begin arriving in countries in late 2025 with service delivery planned to start in early 2026.
Read
- Getting PrEP Rollout Right This Time
This report examines key insights from the rollout of oral PrEP and early introduction of injectable cabotegravir (CAB) and the dapivirine vaginal ring (DVR) to inform a faster, smarter and more equitable introduction of future HIV prevention tools, including long-acting injectable PrEP, such as lenacapavir. - Now What With Injectable LEN For PrEP?
This fact sheet is intended to help outline what is actually known – and not – and what needs to happen next.
- Gears of Lenacapavir for PrEP Rollout
This document outlines a focused plan for LEN for PrEP rollout over the next few years, specifying priorities by stakeholder and evaluating volume and pricing strategies. The report as a whole details a coordinated response to this historic opportunity to ensure rapid implementation, equitable access, and sustainable impact.
- From Clinical Trial Efficacy to Public Health Impact: A Plan for Accelerating Access to Injectable Lenacapavir for PrEP
This plan provides a broad view of all the moving parts and identifies actions and actors responsible for ensuring time is not wasted and opportunity not squandered.
- The Lens on LEN: The Basics on Injectable Lenacapavir as PrEP
This advocates’ primer provides background on lenacapavir for PrEP and its trials; a summary of the early findings of PURPOSE 1 & 2; key questions and next steps.
- Advocates’ Guide to Lenacapavir
This wide-ranging slide deck gives a complete overview of lenacapavir — showing the overall prevention product pipeline, describes lenacapavir, compares it to other options, discusses the trials testing the product, next steps, and links to advocacy resources.
In the News
- AVAC Applauds Agreements to Accelerate Market Development for Lenacapavir for PrEP.
Read AVAC’s statement on what the Gates Foundation and Unitaid’s commitments to accelerate the development of, access to and price reduction for generic versions of LEN mean for the field and what needs to happen next. - Opinion: The world has a new HIV prevention drug. Let’s use it.
There is hope for a new 5 by 3 global target of getting 5 million people on long-acting PrEP to prevent HIV by 2030.
- Access Questions Hang Over Gilead’s HIV Shot
“For all of the wonders of product development, PrEP has not even begun to have the impact we need it to,” said Mitchell Warren, AVAC’s executive director in this Axios news story. - AVAC Calls on Gilead and Global Stakeholders to Accelerate Access to Generic Lenacapavir Following License Agreements
Read AVAC’s statement welcoming Gilead Sciences’ announcement in granting multiple, non-exclusive licenses to generic manufacturers to produce lenacapavir, their investigational twice-a-year injectable for PrEP, while it is still in clinical trials.
Listen
Podcast: Lenacapavir: The case for investing in delivering HIV prevention
This episode of PxPulse goes deep on LEN for PrEP. Recorded just days before Gilead’s announcement that PURPOSE 2 also found very high efficacy, Dr. Flavia Kiweewa, a principal investigator of PURPOSE 1, the first trial to announce efficacy, lays out the research findings and what they mean. And Chilufya Kasanda Hampongo of Zambia’s Treatment Advocacy and Literacy Campaign and Mitchell Warren of AVAC talk about how to change a long history of squandered opportunities to get rollout right.
Watch
- The Scientific Journey of Lenacapavir: From basic science to clinical development to impact
Hosted by AVAC, this webinar explored how US support from NIH for basic science and South Africa’s clinical research infrastructure made possible the development of lenacapavir for PrEP (LEN), a discovery in HIV prevention that went on to be named Science magazine’s 2024 Breakthrough of the Year. - Update on Injectable Lenacapavir for PrEP
AVAC hosted a webinar focused on updates for the PURPOSE trials for injectable lenacapavir for PrEP. Gilead provided an overview of the PURPOSE 1 and 2 trial results and insight into the status of PURPOSE 3, 4, and 5. This was be an opportunity for civil society to hear from Gilead directly. - Breaking Ground: Expanding Access to Lenacapavir
This webinar hosted by Unitaid Advocates Network brought together global health experts, community advocates, and civil society organizations to discuss the challenges and opportunities in ensuring equitable access to Lenacapavir. - AVAC Welcomes FDA Approval of LEN
AVAC’s executive director, Mitchell Warren explains this historic milestone and what’s needed to ensure it’s rolled out with speed, scale, and equity.