Building on the 2024 People’s Research Agenda priorities for MPTs, this 2025 update captures how the field has evolved in the past year.
State of the Field: PRA 2025 Update
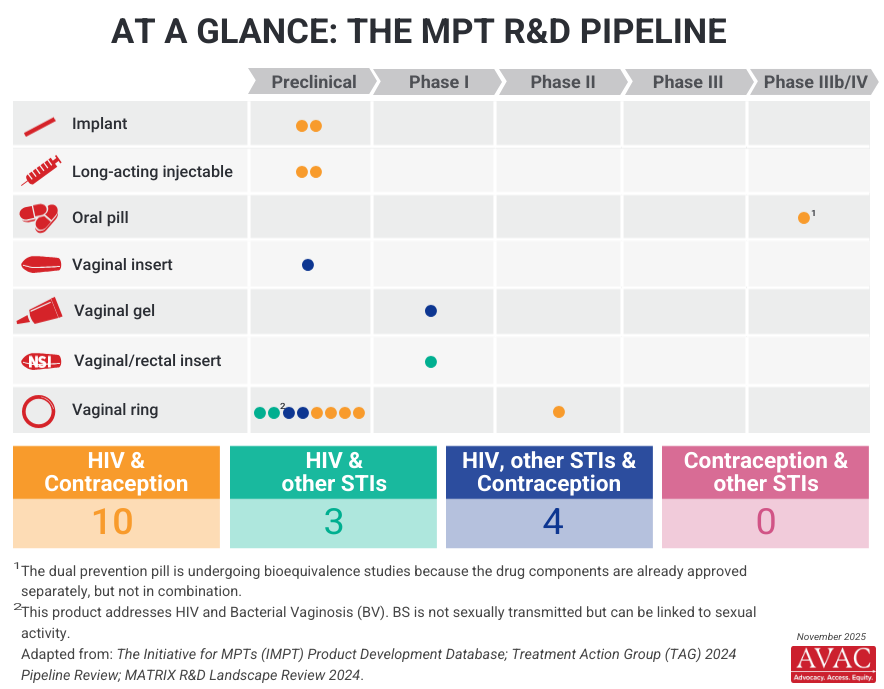
The 2025 MPT R&D portfolio remains heavily weighted toward early discovery and preclinical research, with few candidates beyond Phase I/II readiness:
- The most advanced MPT candidate in 2025 is the Dual Prevention Pill (DPP), which was submitted to the WHO for pre-qualification in 2025. If approved, the DPP could be introduced in 2026. The HPTN 104 Phase IIb trial will evaluate adherence and acceptability of the DPP. This trial begins recruitment in 2026 and is expected to conclude in 2028.
- A significant number of products in the current MPT pipeline are designed for cisgender women, with very few products under development for gender-diverse populations, highlighting ongoing gaps in R&D.
- Cabotegravir is being tested in combination with levonorgestrel. This combination long-acting injectable, currently in animal studies, remains active under an NIH award aimed at three-month injectable formulation. Preclinical results are expected in 2026, with potential progression to further clinical testing, depending on sustained funding.
- A cabotegravir + levonorgestrel pellet implant was deprioritized due to advancements in long-acting injectables.
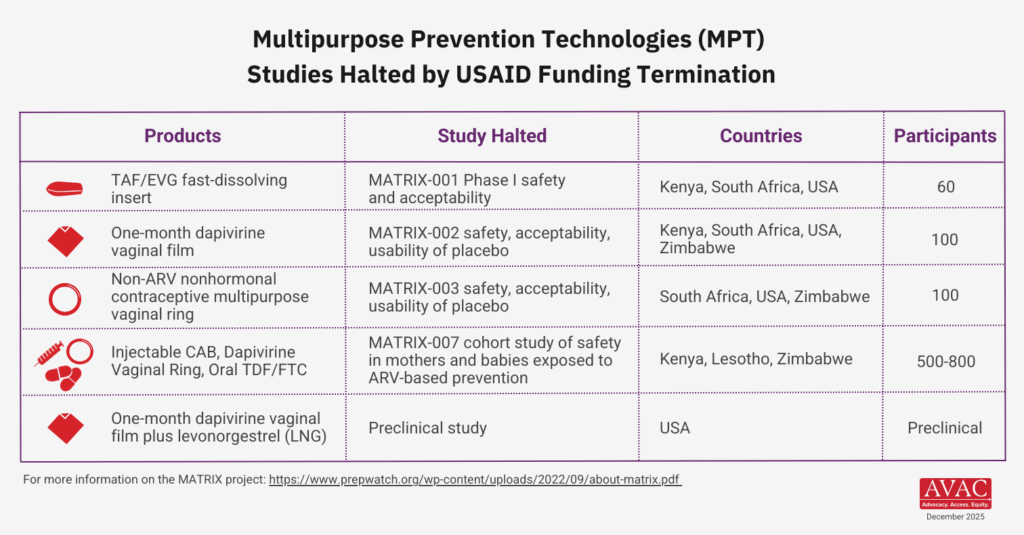
- The MATRIX 001 study of the elvitegravir + tenofovir (TAF) vaginal insert has been completed, but critical data analysis was delayed due to USAID funding cuts. Additional funding has since been secured, and results are anticipated by mid-2026. Developers are currently seeking funding to support Phase II advancement.
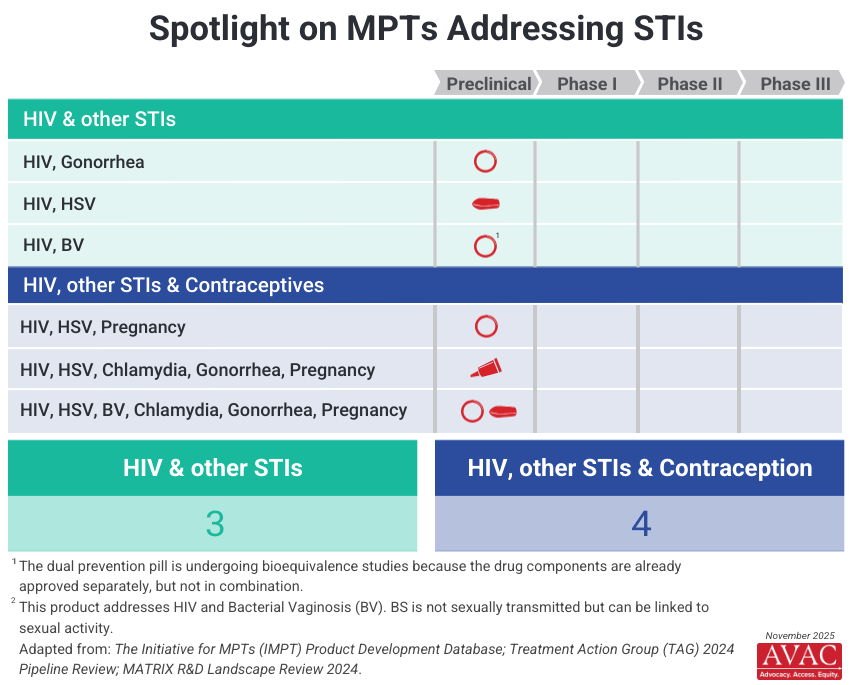
- Dapivirine continues to feature prominently in MPT research, being tested in combination with levonorgestrel and other active ingredients such as metronidazole, pritelivir, copper, and zinc, primarily through the vaginal ring platform.
- The MATRIX 002 study, which investigated a dapivirine + levonorgestrel extended-release monthly film, was terminated due to funding constraints. Developers are exploring alternative funding.
- On-demand, multipurpose products were hardest hit by cuts to USAID.
The field faces a shrinking and shifting resource base that has left the future of most MPTs in development uncertain. Regulatory pathways for MPTs also continue to present questions, as combination products that integrate HIV with contraceptives and/or prevention of sexually transmitted infections (STIs) require collaboration across therapeutic areas. The PRA underscores that sustaining scientific investment, advancing regulatory clarity, and embedding gender-responsive design across the R&D continuum is critical to preserve the MPT pipeline’s potential to deliver the next generation of integrated prevention technologies.
2025 Gaps and Priorities
GAP 1: Limited pipeline diversity; pipeline dominated by ARV-based products
Priority Actions
- Support a streamlined and diverse pipeline of novel formulations and combination products, beyond ARV-only approaches. Following significant funding cuts, a diverse pipeline of MPTs will provide an effective way of meeting multiple needs and preferences and should be considered a cost-effective research strategy.
- Invest in user-centered innovation that reflects the needs of populations beyond cisgender women, including men, transgender and gender-diverse (TGD) people, and peri/post-menopausal individuals.
- Explore self-administered and pleasure-enhancing MPTs to address stigma, improve acceptability, and increase demand.
GAP 2: Funding cuts and stalled progress for next-generation MPT candidates
Priority Actions
- Develop an investment case for key candidates that have been discontinued due to funding cuts, such as the paused elvitegravir + tenofovir fast-dissolving insert (MATRIX-001), the dapivirine + levonorgestrel monthly vaginal film (MATRIX-002), on-demand MPTs, and early-stage injectable MPTs.
- Advocate for funders and developers to maintain investment in a diverse portfolio of MPT studies across diverse delivery platforms (rings, injectables, implants, pills) to ensure continuity, protect user choice, and meet the diverse user requirements.
GAP 3: Unclear manufacturing and regulatory pathways for MPTs
Priority Actions
- Define clear regulatory guidance for products that combine HIV prevention with contraception or STI protection to avoid delays and fragmented approval processes.
- Engage regulators, WHO, and community advocates early to shape pathways that center user safety, affordability, and equity in MPT introduction.
- Leverage lessons from current efforts to prepare the market and manufacturing of the DPP as the first ARV-based MPT.
- Push for more integrated and efficient health systems, leveraging ongoing discussions on health integration and rollout—particularly within sexual and reproductive health (SRH), family planning, and STI services—to ensure effective delivery and normalization of MPTs.
Resources
Navigation
⮐ Return to the 2025 People’s Research Agenda
Previous Edition of the People’s Research Agenda
